Understanding the periodic table through the lens of the volatile Group I metals
- Written by Erwin Boschmann, Professor Emeritus of Chemistry & Chemical Biology, IUPUI
The news broke that a railroad car, loaded with pure sodium, had just derailed and was spilling its contents. A television reporter called me for an explanation of why firefighters were not allowed to use water on the flames bursting from the mangled car. While on the air I added some sodium to a bit of water in a petri dish and we observed the vicious reaction. For further dramatic effect, I also placed some potassium into water and astonished everyone with the explosive bluish flames.
Because Group I metals, also known as alkali metals, are very reactive, like the sodium from the rail car or the potassium, they are not found in nature in pure form but only as salts. Not only are they very reactive, they are soft and shiny, can easily be cut even with a dull knife and are the most metallic of all known elements.
I am a chemist who spent his career building new molecules, sometimes using Group I elements. By studying the behavior and trends of Group I elements, we can get a glimpse of how the periodic table is arranged and how to interpret it.
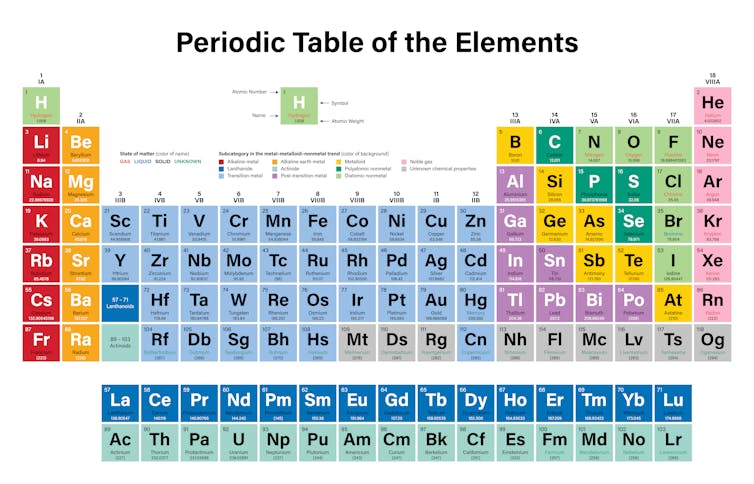 Periodic Table of the Elements. The Group I metals are on the far left colored red.
Humdan/Shutterstock.com[1]
Periodic Table of the Elements. The Group I metals are on the far left colored red.
Humdan/Shutterstock.com[1]
The basics
The arrangement of the periodic table and the properties of each element in it is based of the atomic number and the arrangement of the electrons orbiting the nucleus. The atomic number describes the number of protons in the nucleus of the element. Hydrogen’s atomic number is 1, helium’s is 2, lithium’s is 3 and so on.
Each of the 18 columns in the table is called a group or a family. Elements in the same group share similar properties. And the properties can be assumed based on the location within the group. Going from the top of Group I to the bottom, for example, the atomic radii – the distance from the nucleus to the outer electrons – increases. But the amount of energy needed to rip off an outer electron decreases going from the top to the bottom because the electrons are farther from the nucleus and not held as tightly.
This is important because how elements interact and react with each other depends on their ability to lose and gain electrons to make new compounds.
The horizontal rows of the table are called periods. Moving from the left side of the period to the right, the atomic radius becomes smaller because each element has one additional proton and one additional electron. More protons means that electrons are pulled in more tightly toward the nucleus. For the same reason electronegativity – the degree to which an element tends to gain electrons – increases from left to right.
The force required to remove the outermost electron, known as the ionization potential, also increases from the left-hand side of the table, which has elements with a metallic character, to the right side, which are nonmetals.
Electronegativity decreases from the top of the column to the bottom. The melting point of the elements within a group also decreases from the top to the bottom of a group.
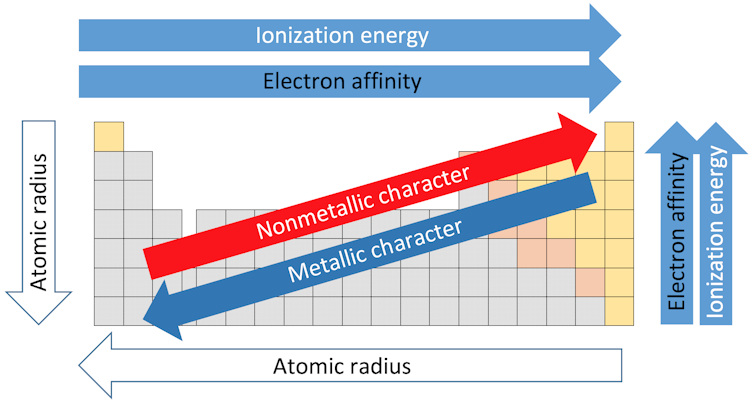 Trends of the periodic table.
Sandbh/Wikipedia, CC BY-SA[2][3]
Trends of the periodic table.
Sandbh/Wikipedia, CC BY-SA[2][3]
Applying the basics to Group I elements
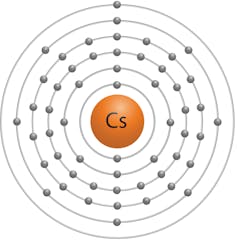 The outermost electron surrounding the Cesium atom is far from the nucleus and thus easy to remove. That makes cesium highly reactive.
gstraub/Shutterstock.com[4]
The outermost electron surrounding the Cesium atom is far from the nucleus and thus easy to remove. That makes cesium highly reactive.
gstraub/Shutterstock.com[4]
As its name implies, Group I elements occupy the first column in the periodic table. Each element starts a new period. Lithium is at the top of the group and is followed by sodium, Na; potassium, K; rubidium, Rb; cesium, Cs and ends with the radioactive francium, Fr. Because it is highly radioactive, virtually no chemistry is performed with this element.
Because each element in this column has a single outer electron in a new shell, the volumes of these elements are large and increase dramatically when moving from the top to the bottom of the group.
Of all the Group I elements, cesium has the largest volumes because the outermost single electron is loosely held.
In spite of these trends, the properties of the elements of Group I are more similar to each other than those of any other group.
Alkali metals through history
Using chemical properties as his guide, Russian chemist Dimitri Mendeleev correctly ordered the first Group I elements into his 1869 periodic table. It is called periodic because every eighth element repeats the properties of the one above it in the table. After arranging all of the then known elements, Mendeleev took the bold step of leaving blanks where his extrapolation of chemical properties showed that an element should exist. Subsequent discovery of these new elements proved his prediction correct.
 Fireworks owe their vivid colors to the Group I metals.
elena_prosvirova/Shutterstock.com[5]
Fireworks owe their vivid colors to the Group I metals.
elena_prosvirova/Shutterstock.com[5]
Some alkali metals have been known and put to good use long before Mendeleev created the periodic table. For instance, the Old Testament mentions salt – a combination of the alkali metal sodium with chlorine – 31 times. The New Testament refers to it 10 times and calls sodium carbonate “neter” and potassium nitrate “saltpeter.”
People have known since antiquity that wood ashes produce a potassium salt which, when combined with animal fat, will yield soap. Samuel Hopkins obtained the first U.S. patent on July 31, 1790, for soap under the new patent statute just signed into law by President George Washington a few months earlier.
The pyrotechnic industry loves these Group I elements for their vibrant colors and explosive nature. Burning lithium produces a vivid crimson red color; sodium a yellow one; potassium lilac; rubidium red; and cesium violet. These colors are produced as electrons jump from their home environment orbiting the nucleus and returning back again.
The cesium atomic clock, the most accurate timepiece ever developed, functions by measuring the frequency of cesium electrons jumping back and forth between energy states. Clocks based on electrons jumping provide an extremely precise way to count seconds.
Sodium metal reacts with water to produce sodium hydroxide, hydrogen gas and energy.Other applications include sodium vapor lamps and lithium batteries.
In my own research I have used Group I metals as tools to perform other chemistry. Once I was in need of absolutely dry alcohol, and the driest I could buy still contained minute traces of water. The only way to get rid of the last remnant of water was by treating the water-containing alcohol with sodium – a rather dramatic way to remove water.
The alkali elements not only occupy the first column in the periodic table, but they also show the most reactivity of all groups in the entire table and have the most dramatic trends in volume and ionization potential, while maintaining great similarity among themselves.
References
- ^ Humdan/Shutterstock.com (www.shutterstock.com)
- ^ Sandbh/Wikipedia (upload.wikimedia.org)
- ^ CC BY-SA (creativecommons.org)
- ^ gstraub/Shutterstock.com (www.shutterstock.com)
- ^ elena_prosvirova/Shutterstock.com (www.shutterstock.com)
Authors: Erwin Boschmann, Professor Emeritus of Chemistry & Chemical Biology, IUPUI

