Where does the controversial finding that adult human brains don't grow new neurons leave ongoing research?
- Written by Janice R. Naegele, Alan M Dachs Professor of Science, Professor of Biology, Neuroscience and Behavior, Wesleyan University
Scientists have known for about two decades that some neurons – the fundamental cells in the brain that transmit signals – are generated throughout life[1]. But now a controversial new study from the University of California, San Francisco, casts doubt on whether many neurons are added to the human brain after birth[2].
As a translational neuroscientist[3], this work immediately piqued my interest. It has direct implications for the research my lab does[4]: We transplant young neurons into damaged brain areas in mice in an attempt to treat epileptic seizures and the damage they’ve caused. Like many labs, part of our work is based on a foundational belief that the hippocampus is a brain region where new neurons are born throughout life.
If the new study is right, and human brains for the most part don’t add new neurons after infancy, researchers like me need to reconsider the validity of the animal models we use to understand various brain conditions – in my case temporal lobe epilepsy. And I suspect other labs that focus on conditions including drug addiction, depression and post-traumatic stress disorder are thinking about what the UCSF study means for their investigations, too.
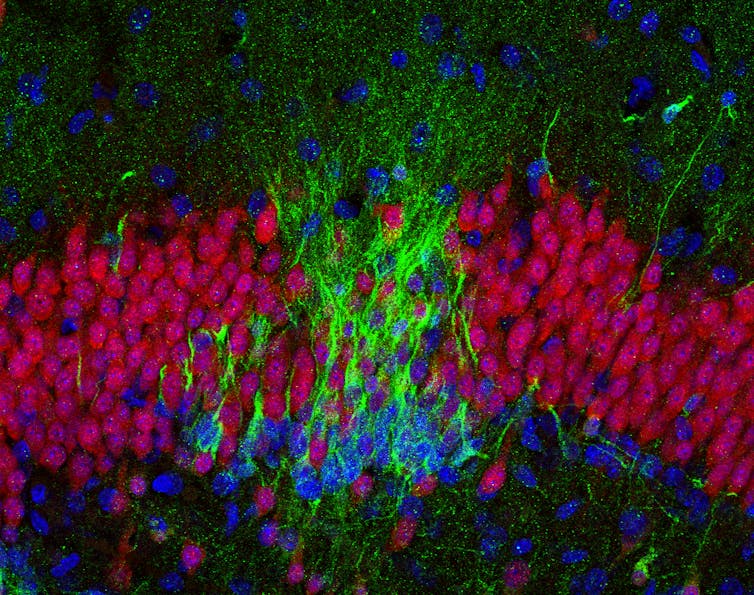 In the brain of a baby who died soon after birth, there are many new neurons (green in this image) in the hippocampus.
Sorrells et al, CC BY-ND[5][6]
In the brain of a baby who died soon after birth, there are many new neurons (green in this image) in the hippocampus.
Sorrells et al, CC BY-ND[5][6]
When and where are new neurons born?
No doubt, the adult human brain is able to learn throughout life and to change and adapt – a capability brain scientists call neuroplasticity, the brain’s ability to reorganize itself by rewiring connections[7]. Yet, a central dogma in the field of neuroscience for nearly 100 years had been that a child is born with all the neurons she will ever have[8] because the adult brain cannot regenerate neurons.
Just over half a century ago, researchers devised a way to study proliferation of cells in the mature brain, based on techniques to incorporate a radioactive label[9] into new cells as they divide. This approach led to the startling discovery in the 1960s that rodent brains actually could generate new neurons[10].
Neurogenesis – the production of new neurons – was previously thought to only occur during embryonic life, a time of extremely rapid brain growth and expansion, and the rodent findings were met with considerable skepticism. Then researchers discovered that new neurons are also born throughout life in the songbird brain[11], a species scientists use as a model for studying vocal learning. It started to look like neurogenesis plays a key role in learning and neuroplasticity – at least in some brain regions in a few animal species.
Even so, neuroscientists were skeptical that many nerve cells could be renewed in the adult brain; evidence was scant that dividing cells in mammalian brains produced new neurons, as opposed to other cell types. It wasn’t until researchers extracted neural stem cells from adult mouse brains and grew them in cell culture that scientists showed these precursor cells could divide and differentiate into new neurons[12]. Now it is generally well accepted that neurogenesis takes place in two areas of the adult rodent brain: the olfactory bulbs, which process smell information, and the hippocampus, a region characterized by neuroplasticity that is required for forming new declarative memories.
Adult neural stem cells cluster together in what scientists call niches – hotbeds for cultivating the birth and growth of new neurons[13], recognizable by their distinctive architecture. Despite the mounting evidence for regional growth of new neurons, these studies underscored the point that the adult brain harbors only a few stem cell niches and their capacity to produce neurons is limited to just a few types of cells.
With this knowledge, and new tools for labeling proliferating cells and identifying maturing neurons, scientists began to look for postnatal neurogenesis in primate and human brains.
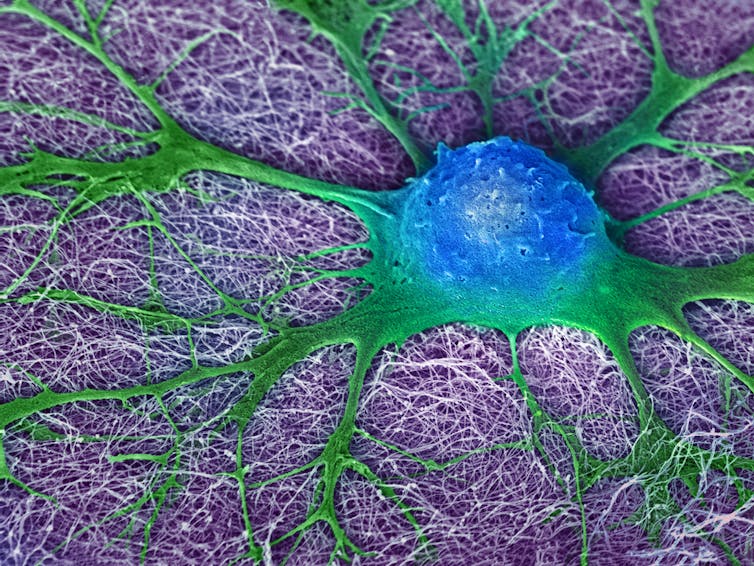 A mouse neural stem cell (blue and green) grows in a lab dish. Can human brain cells do what rodent brain cells do?
Mark McClendon, Zaida Alvarez Pinto, Samuel I. Stupp, Northwestern University, Evanston, IL, CC BY-NC[14][15]
A mouse neural stem cell (blue and green) grows in a lab dish. Can human brain cells do what rodent brain cells do?
Mark McClendon, Zaida Alvarez Pinto, Samuel I. Stupp, Northwestern University, Evanston, IL, CC BY-NC[14][15]
What’s happening in adult human brains?
Many neuroscientists believe that by understanding the process of adult neurogenesis we’ll gain insights into the causes of some human neurological disorders. Then the next logical step would be trying to develop new treatments harnessing neurogenesis for conditions such as Alzheimer’s disease or trauma-induced epilepsy. And stimulating resident stem cells in the brain to generate new neurons is an exciting prospect for treating neurodegenerative diseases.
Because neurogenesis and learning in rodents increases with voluntary exercise[16] and decreases with age[17] and early life stress[18], some workers in the field became convinced that older people might be able to enhance their memory as they age by maintaining a program of regular aerobic exercise[19].
However, obtaining rigorous proof for adult neurogenesis in the human and primate brain has been technically challenging – both due to the limited experimental approaches and the larger sizes of the brains, compared to reptiles, songbirds and rodents.
Researchers injected a compound found in DNA, nicknamed BrdU to identify brand new neurons in human adult hippocampus[20] – but the labeled cells were extremely rare. Other groups demonstrated that adult human brain tissue obtained during neurosurgery contained stem cell niches that housed progenitor cells that could generate new neurons in the lab[21], showing that these cells had an inborn neurogenic capacity, even in adults.
But even when scientists saw evidence for new neurons in the brain, they tended to be scarce. Some neurogenesis experts were skeptical that evidence based on incorporating BrdU into DNA was a reliable method for proving that new cells were actually being born through cell division, rather than just serving as a marker for other normal cell functions[22].
Further questions about how long human brains retain the capacity for neurogenesis arose in 2011, with a study that compared numbers of newborn neurons migrating[23] in the olfactory bulbs of infants versus older individuals up to 84 years of age. Strikingly, in the first six months of life, the baby brains contained lots of chains of young neurons migrating into the frontal lobes[24], regions that guide executive function, long-range planning and social interactions. These areas of the human cortex are hugely increased in size and complexity compared to rodents and other species. But between 6 to 18 months of age, the migrating chains dwindled to a thin stream. Then, a very different pattern emerged: Where the migrating chains of neurons had been in the infant brain, a cell-free gap appeared, suggesting that neural stem cells become depleted during the first six months of life.
Questions still lingered about the human hippocampus and adult neurogenesis as a source for its neuroplasticity. One group came up with a clever approach based on radiocarbon dating. They measured how much atmospheric ¹⁴C – a radioactive isotope derived from nuclear bomb tests – was incorporated into people’s DNA. This method suggested that as many as 700 new cells are added to the adult human hippocampus every day[25]. But these findings were contradicted by a 2016 study that found that the neurogenic cells in the adult hippocampus could only produce non-neuronal brain cells called microglia[26].
Rethinking neurogenesis research
Now the largest and most comprehensive study conducted to date presents even stronger evidence[27] that robust neurogenesis doesn’t continue throughout adulthood in the human hippocampus – or if it does persist, it is extremely rare. This work is controversial and not universally accepted. Critics have been quick to cast doubt on the results[28], but the finding isn’t totally out of the blue.
So where does this leave the field of neuroscience? If the UCSF scientists are correct, what does that mean for ongoing research in labs around the world?
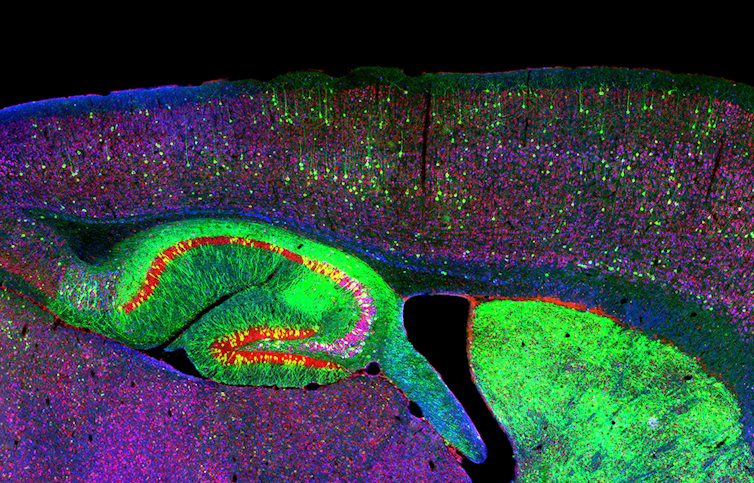 It’s much easier to work with rodent brains than human ones. This is a stained image of the hippocampus and neurons of a mouse with neurodegenerative disease.
NICHD/I. Williams, CC BY[29][30]
It’s much easier to work with rodent brains than human ones. This is a stained image of the hippocampus and neurons of a mouse with neurodegenerative disease.
NICHD/I. Williams, CC BY[29][30]
Because lots of studies of neurological diseases are done in mice and rats, many scientists are invested in the possibility that adult neurogenesis persists in the human brain, just as it does in rodents. If it doesn’t, how valid is it to think that the mechanisms of learning and neuroplasticity in our model animals are comparable to those in the human brain? How relevant are our models of neurological disorders for understanding how changes in the hippocampus contribute to disorders such as the type of epilepsy I study?
In my lab, we transplant embryonic mouse or human neurons into the adult hippocampus in mice, after damage caused by epileptic seizures[31]. We aim to repair this damage and suppress seizures by seeding the mouse hippocampus with neural stem cells that will mature and form new connections. In temporal lobe epilepsy, studies in adult rodents suggest that naturally occurring hippocampal neurogenesis is problematic. It seems that the newborn hippocampal neurons become highly excitable and contribute to seizures. We’re trying to inhibit these newborn hyperexcitable neurons with the transplants. But if humans don’t generate new hippocampal neurons, then maybe we’re developing a treatment in mice for a problem that has a different mechanism in people.
Perhaps our species has evolved separate mechanisms for neuroplasticity, distinct from those used by species such as rats and mice. One possibility is that there are other sites in the human brain where neurogenesis occurs - its a big structure and more exploration will be necessary. If it turns out to be true that the human brain has a diminished capacity for neurogenesis after birth, the finding will have important implications for how neuroscientists like me think about tackling brain disorders.
Perhaps most importantly, this work underscores how crucial it is to learn how to increase the longevity of the neurons we do have, born early in life, and how we might replace or repair neurons that become damaged.
References
- ^ generated throughout life (www.jneurosci.org)
- ^ neurons are added to the human brain after birth (doi.org)
- ^ As a translational neuroscientist (scholar.google.com)
- ^ research my lab does (naegelelab.research.wesleyan.edu)
- ^ Sorrells et al (doi.org)
- ^ CC BY-ND (creativecommons.org)
- ^ brain’s ability to reorganize itself by rewiring connections (theconversation.com)
- ^ born with all the neurons she will ever have (doi.org)
- ^ techniques to incorporate a radioactive label (doi.org)
- ^ rodent brains actually could generate new neurons (doi.org)
- ^ born throughout life in the songbird brain (www.pnas.org)
- ^ divide and differentiate into new neurons (doi.org)
- ^ hotbeds for cultivating the birth and growth of new neurons (doi.org)
- ^ Mark McClendon, Zaida Alvarez Pinto, Samuel I. Stupp, Northwestern University, Evanston, IL (www.flickr.com)
- ^ CC BY-NC (creativecommons.org)
- ^ increases with voluntary exercise (doi.org)
- ^ decreases with age (doi.org)
- ^ early life stress (doi.org)
- ^ regular aerobic exercise (doi.org)
- ^ identify brand new neurons in human adult hippocampus (doi.org)
- ^ could generate new neurons in the lab (doi.org)
- ^ marker for other normal cell functions (www.jneurosci.org)
- ^ newborn neurons migrating (doi.org)
- ^ migrating into the frontal lobes (doi.org)
- ^ 700 new cells are added to the adult human hippocampus every day (doi.org)
- ^ could only produce non-neuronal brain cells called microglia (doi.org)
- ^ presents even stronger evidence (theconversation.com)
- ^ quick to cast doubt on the results (www.statnews.com)
- ^ NICHD/I. Williams (www.flickr.com)
- ^ CC BY (creativecommons.org)
- ^ into the adult hippocampus in mice, after damage caused by epileptic seizures (doi.org)
Authors: Janice R. Naegele, Alan M Dachs Professor of Science, Professor of Biology, Neuroscience and Behavior, Wesleyan University

