Skipping a few thousand years: Rapid domestication of the groundcherry using gene editing
- Written by Nathan T. Reem, Postdoctoral Researcher in the Boyce Thompson Institute, Cornell University
Shopping in your supermarket’s produce section is like strolling through a museum of humanity’s greatest inventions. Perfect ears of golden sweet corn; tomatoes of different sizes, shapes and colors; and spicy jalapeño peppers are all a testament to human ingenuity. You may not consider food an invention, but nearly all foods we eat are the product of thousands of years of constant breeding and selection.
In the distant past, when our ancestors transitioned from hunter-gatherers to an agrarian lifestyle, they began domesticating plants by breeding them for characteristics they found desirable – bigger, tastier fruits and more compact growth. The wild ancestors of domesticated crops[1] looked much different than the foods we eat today: They had smaller, sometimes inedible fruits; the plants grew in a sprawling growth pattern; and they scattered their seeds or dropped their fruit to the ground in order to ensure the survival of their species. To put it bluntly, you wouldn’t want these wild plants in your garden, or on your dinner plate.
The process of domestication resulted in the crops people grow and eat today, but it is a time- and labor-intensive process. Our lab, led by Joyce Van Eck[2], wanted to accelerate the domestication of the groundcherry, a semi-domesticated orphan crop, using modern gene editing techniques. Orphan crops do not grow well in large-scale agricultural production because they possess many undesirable characteristics such as sprawling growth and fruit drop.
We chose to work on groundcherry because it is a relative of domesticated tomato. We know a lot about tomato genetics and are able to compare a particular gene in domesticated tomato with its counterpart in the wild groundcherry to determine what edits need to be made. We have also crowdsourced local growers and farmers to learn which traits needed improvement and which ones were most valuable for agricultural production. Using this critical information gleaned from growers, we then used gene editing technology known as CRISPR/Cas9 to improve groundcherries.
 A typical groundcherry plant. Inset: groundcherry husks and fruit (dime for scale).
Nathan T. Reem
A typical groundcherry plant. Inset: groundcherry husks and fruit (dime for scale).
Nathan T. Reem
A neglected fruit
Although you likely won’t find them in your grocery store, you may have seen groundcherries for sale at your local farmer’s market. The groundcherry is a wild relative of the tomatillo and, much like the tomatillo, its fruits are encased within a papery husk that protects the fruit from spoiling. The berry inside the husk is small – marble-sized – but delivers a big citrusy flavor. A source of antioxidants, vitamins A, B and C, and other nutrients, these small berries are exclusively grown in small-scale farms and home gardens. Based on the groundcherry’s wild growth habit and small size of fruit, we identified it as an underutilized crop. Our current research has been focused on how to incorporate groundcherry into the current food system.
 Wild groundcherries growing on a small farm.
Esperanza Shenstone, CC BY-SA[3]
Wild groundcherries growing on a small farm.
Esperanza Shenstone, CC BY-SA[3]
Commercial production of the groundcherry (Physalis pruinosa) is virtually nonexistent, a void that can at least partially be attributed to the plant’s unruly growth. With its long sprawling branches, the groundcherry requires extensive management to tame its growth. Its branches are adorned with husk-covered fruits that fall to the ground, often before ripening. This makes harvesting the fruits a labor-intensive process, and raises food safety concerns if the fruits come in contact with soil microorganisms that can cause food-borne illnesses.
A critical element of our groundcherry improvement project was crowdsourcing the wisdom of New York state citizen scientists and farmers to identify groundcherry characteristics or traits that needed improvement. Volunteer home-gardeners and farmers across different USDA hardiness zones collaborated with us by growing several groundcherry varieties and provided feedback on characteristics such as flowering time, fruit size, flavor and fruit drop. We used this critical feedback for improve this fruit.
Taming the wild plants
To improve traits in crops, plant breeders have largely relied on the natural mutations that occur in all living organisms. These natural mutation events change gene sequences and thus modify traits, but they are rare. Before gene editing, there were few tools to speed the breeding process. One of these, called ethyl methanesulfonate (EMS), is a powerful carcinogen and used to randomly mutate DNA of thousands of plants. The downside is that all of the mutated plants must be carefully assessed to select those with mutations in the genes breeders wished to modify.
This process, still in use today, is messy and time-consuming; there is no way to control which genes are, or aren’t, mutated, and screening thousands of plants can take time.
CRISPR/Cas9 is a powerful gene editing tool that can be used to cause mutations in DNA more precisely than the EMS-induced random mutations. Rather than waiting for random mutations or evaluating thousands of mutagenized plants, CRISPR/Cas9 can accelerate breeding and domestication of crops[4] with greater specificity than any other technology. Fortunately, many of the traits associated with domestication, including fruit size and growth habit, are the result of natural mutations and rearrangements of DNA that ultimately change the function of the genes controlling these traits. CRISPR/Cas9 allows us to copy these mutations from the tomato and replicate them in the groundcherry.
Along with our collaborator Zach Lippman at Cold Spring Harbor Laboratory[5], we recently published our first attempts at accelerating domestication of groundcherry in the journal Nature Plants[6]. Our first priority was to tame the wild growth.
In tomato, a natural mutation in the SELF PRUNING (SP) gene, which represses flowering, results in plants that have more manageable growth[7]. We hoped to see the same response when we “CRISPR’ed” groundcherries, and found that the plants with mutated SP grew with a much more compact structure. Specifically, the branches of these plants were much shorter than their unedited counterparts. This more diminutive growth habit is preferable for larger-scale agricultural settings, because more compact plants can be grown and harvested more easily.
 Left: groundcherry with CRISPR’ed SP. Right: unedited groundcherry.
Nathan T. Reem
Left: groundcherry with CRISPR’ed SP. Right: unedited groundcherry.
Nathan T. Reem
We targeted one more well-studied gene in groundcherry, called CLAVATA1 (CLV1), which directly controls fruit size[8]. In tomato, mutations in CLV1 result in larger fruits. Because groundcherry fruits are rather small, we tried to increase fruit size by mutating CLV1 with CRISPR/Cas9.
At first glance, groundcherry plants with mutated CLV1 looked the same as their unedited counterparts. However, fruits from CLV1-mutant plants were larger, weighing 20 percent more after mutating this single gene. CLV1 is just one of many genes controlling fruit size. We expect that mutating more of these genes will enable us to create larger fruit in a short time. The process of CRISPR'ing a plant gene, such as CLV1 and SP, takes only about a year, whereas traditional breeding usually requires much more time and effort to achieve the same result.
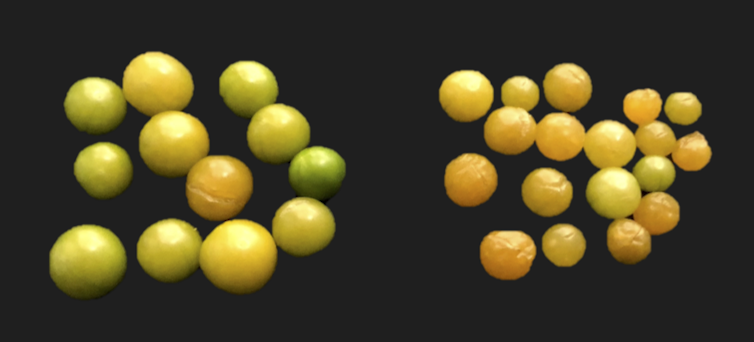 Left: groundcherry fruits with CRISPR’ed CLV1. Right: unedited groundcherries.
Nathan T. Reem
Left: groundcherry fruits with CRISPR’ed CLV1. Right: unedited groundcherries.
Nathan T. Reem
In order to fully domesticate and improve groundcherry, we plan to study more genes associated with characteristics that would make it more attractive crops for farmers to grow and consumers to purchase. Currently, we are focusing on genes that have the potential to correct fruit drop, influence fruit flavor and nutrition, and increase fruit size further.
Ultimately, we envision creating a more compact groundcherry plant with larger, more nutrient-laden fruits that remain on the plant. To do so, CRISPR/Cas9 mutations of all the genes controlling these traits will be combined into a single plant to create a fully domesticated groundcherry worthy of growing in farmers’ fields and stocking grocery store shelves. Importantly, the groundcherry isn’t the only wild plant that can be domesticated. CRISPR/Cas9 can be applied to virtually any plant species, so in the future more wild species may be domesticated much the same way we have achieved here.
So, the next time you go shopping for groceries, pay attention to the produce aisle. Appreciate the efforts of our ancestors that took thousands of years to invent the foods we know today, and think how gene editing will help achieve this in a fraction of the time.
References
- ^ The wild ancestors of domesticated crops (doi.org)
- ^ Joyce Van Eck (btiscience.org)
- ^ CC BY-SA (creativecommons.org)
- ^ CRISPR/Cas9 can accelerate breeding and domestication of crops (doi.org)
- ^ Zach Lippman at Cold Spring Harbor Laboratory (lippmanlab.labsites.cshl.edu)
- ^ in the journal Nature Plants (doi.org)
- ^ more manageable growth (www.ncbi.nlm.nih.gov)
- ^ controls fruit size (doi.org)
Authors: Nathan T. Reem, Postdoctoral Researcher in the Boyce Thompson Institute, Cornell University

