Anti-cancer CAR-T therapy reengineers T cells to kill tumors – and researchers are expanding the limited types of cancer it can target
- Written by Gregory Allen, Assistant Professor of Medicine, University of California, San Francisco
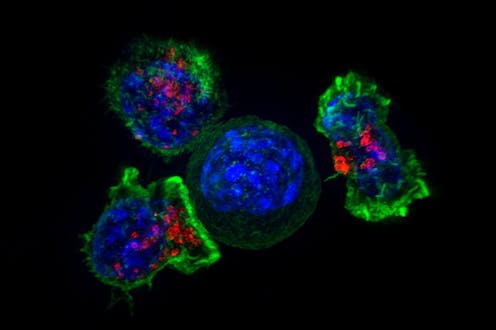
Teaching the body’s immune cells to recognize and fight cancer is one of the holy grails in medicine. Over the past two decades, researchers have developed new immunotherapy drugs[1] that stimulate a patient’s immune cells to significantly shrink or even eliminate tumors. These treatments often focus on increasing the cancer-killing ability of cytotoxic T cells[2]. However, these treatments appear to only work for the small group of patients[3] who already have T cells within their tumors. One 2019 study estimated that under 13%[4] of cancer patients responded to immunotherapy.
To bring the benefits of immunotherapy to more patients, scientists have turned to synthetic biology[5], a new field of study that seeks to redesign nature with new and more useful functions. Researchers have been developing a novel type of therapy that directly gives patients a new set of T cells engineered to attack tumors: chimeric antigen receptor T cells, or CAR-T cells for short.
As an oncology physician and researcher[6], I believe that CAR-T cell therapy has the potential to transform cancer treatment. It’s already being used to treat lymphoma[7] and multiple myeloma[8], and has shown remarkable response rates where other treatments have failed.
However, similar success against certain types of tumors such as lung or pancreatic cancer has been slower to develop because of the unique obstacles they put up against T cells. In our newly published research[9], my colleagues and I have found that adding a synthetic circuit to CAR-T cells could potentially help them bypass the barriers that tumors put up and enhance their ability to eliminate more types of cancer.
CAR-T cell therapy is currently only used for certain types of blood cancers.How does CAR-T cell therapy work?
CAR-T cell therapy starts with doctors isolating a patient’s T cells from a sample of their blood. These T cells are then taken back to the lab, where they are genetically engineered to produce a chimeric antigen receptor, or CAR[10].
CARs are synthetic receptors specifically designed to redirect T cells from their usual targets have them recognize and hone in on tumor cells. On the outside of a CAR is a binder that allows the T cell to stick to tumor cells. Binding to a tumor cell activates the engineered T cell to kill and produce inflammatory cytokines[11] proteins that support T cell growth and function and boost their cancer-killing abilities.
These CAR-T cells are then stimulated to divide into large numbers over seven to 10 days, then given back to the patient via infusion. The infusion process usually takes place at a hospital where clinicians can monitor for signs of an overactive immune response[13] against tumors, which can be deadly for the patient.
Driving T cells into solid tumors
While CAR-T cell therapy has seen success in blood cancers, it has faced hurdles when fighting what are called solid tumor cancers like pancreatic cancer and melanoma. Unlike cancers that begin in the blood, these types of cancers grow into a solid mass that produces a microenvironment[14] of molecules, cells and structures that prevent T cells from entering into the tumor and triggering an immune response. Here, even CAR-T cells engineered to specifically target a patient’s unique tumor are unable to access it, suppressing their ability to kill tumor cells.
For the synthetic biology community, the failures of the first generation of CAR-T cell therapy was a call to action to develop a new family of synthetic receptors to tackle the unique challenges solid tumors posed. In 2016, my colleagues in the Lim Lab[15] at the University of California, San Francisco developed a new synthetic receptor that could complement the first CAR design. This receptor, called synthetic Notch receptor, or synNotch[16], is based on the natural form of Notch in the body, which plays an important role in organ development across many species.
Similar to CARs, the outside of synNotch has a binder that allows T cells to stick to tumor cells. Unlike CARs, the inside of synNotch has a protein that is released when a T cell binds to the tumor. This protein, or transcription factor, allows researchers to better control the T cell by inducing it to produce a specific protein.
For example, one of the most useful applications of synNotch thus far has been to use it to ensure that engineered T cells are only activated when bound to a tumor cell and not healthy cells. Because a CAR may bind to both tumor and healthy cells and induce T cells to kill both, my colleagues engineered T cells that are only activated when both synNotch and CAR[17] are bound to the tumor cell. Because T cells now require both CAR and synNotch receptors to recognize tumors, this increases the precision of T cell killing.
Researchers are engineering CAR-T cells to be more precise, and subsequently reduce side effects.We wondered if we could use synNotch to improve CAR-T cell activity against solid tumors by inducing them to produce more of the inflammatory cytokines, such as IL-2, that enable them to kill tumor cells. Researchers have made many attempts to provide extra IL-2 to help CAR-T cells clear tumors. But because these cytokines are highly toxic[18], there is a limit to how much IL-2 a patient can safely tolerate, limiting their use as a drug.
So we designed CAR-T cells to produce IL-2 using synNotch[19]. Now, when a CAR-T cell encounters a tumor, it produces IL-2 within the tumor instead of outside it, avoiding causing harm to surrounding healthy cells. Because synNotch is able to bypass the barriers tumors put up, it is able to help T cells amp up and maintain the amount of IL-2 they can make, allowing the T cells to keep functioning even in a hostile microenvironment.
We tested our CAR-T cells modified with synNotch on mice with pancreatic cancer and melanoma. We found that CAR-T cells with synNotch-induced IL-2 were able to produce enough extra IL-2 to overcome the tumors’ defensive barriers and fully activate, completely eliminating the tumors. While all of the mice receiving synNotch modified CAR-T cells survived, none of the CAR-T-only mice did.
Furthermore, our synNotch modified CAR-T cells were able to trigger IL-2 production without causing toxicity to healthy cells in the rest of the body. This suggests that our method of engineering T cells to produce this toxic cytokine only where it is needed can help improve the effectiveness of CAR-T cells against cancer while reducing side effects.
Next steps
Fundamental questions remain on how this work in mice will translate to people. Our group is currently conducting more studies on using CAR-T cells with synNotch to produce IL-2, with the goal of entering early stage clinical trials to examine its safety and efficacy in patients with pancreatic cancer.
Our findings are one example of how advances in synthetic biology make it possible to engineer solutions to the most fundamental challenges in medicine.
References
- ^ have developed new immunotherapy drugs (doi.org)
- ^ cytotoxic T cells (bio.libretexts.org)
- ^ small group of patients (doi.org)
- ^ under 13% (doi.org)
- ^ synthetic biology (www.genome.gov)
- ^ oncology physician and researcher (scholar.google.com)
- ^ lymphoma (doi.org)
- ^ multiple myeloma (doi.org)
- ^ newly published research (www.science.org)
- ^ chimeric antigen receptor, or CAR (doi.org)
- ^ inflammatory cytokines (www.cancer.org)
- ^ National Cancer Institute (NCI) (visualsonline.cancer.gov)
- ^ overactive immune response (doi.org)
- ^ microenvironment (doi.org)
- ^ Lim Lab (limlab.ucsf.edu)
- ^ synthetic Notch receptor, or synNotch (doi.org)
- ^ both synNotch and CAR (doi.org)
- ^ highly toxic (doi.org)
- ^ produce IL-2 using synNotch (www.science.org)
Authors: Gregory Allen, Assistant Professor of Medicine, University of California, San Francisco

