A new type of chemical bond: The charge-shift bond
- Written by John Morrison Galbraith, Associate Professor of Chemistry, Marist College
 The Abstract features interesting research and the people behind it.
John Morrison Galbraith[1] is an associate professor of chemistry at Marist College who studies chemical bonding, which is the process that holds atoms together to make molecules.
What have you discovered?
Did you take a chemistry course in high school? Did you think it was a boring static field filled with established facts that were determined a long time ago? I’ve done research that shows that the most fundamental of these established “facts,” the nature of the chemical bond, is now being questioned.
You have likely heard of covalent bonds, where electrons are shared between atoms, and ionic bonds, where electrons are completely transferred from one atom to another. But you probably don’t know about a third type of bond, discovered in the early 1990s by Sason Shaik[2] and Philippe Hiberty[3]: the charge-shift bond[4]. I began working with them soon after.
The Abstract features interesting research and the people behind it.
John Morrison Galbraith[1] is an associate professor of chemistry at Marist College who studies chemical bonding, which is the process that holds atoms together to make molecules.
What have you discovered?
Did you take a chemistry course in high school? Did you think it was a boring static field filled with established facts that were determined a long time ago? I’ve done research that shows that the most fundamental of these established “facts,” the nature of the chemical bond, is now being questioned.
You have likely heard of covalent bonds, where electrons are shared between atoms, and ionic bonds, where electrons are completely transferred from one atom to another. But you probably don’t know about a third type of bond, discovered in the early 1990s by Sason Shaik[2] and Philippe Hiberty[3]: the charge-shift bond[4]. I began working with them soon after.
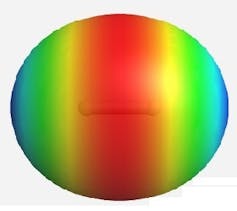 The three types of chemical bonds. Red indicates electron-rich areas and blue indicates electron-deficient areas. (Top) the covalent bond in the hydrogen molecule showing electron build up in the bonding region between two indivual hydrogen atoms.
CC BY-SA[5]
The three types of chemical bonds. Red indicates electron-rich areas and blue indicates electron-deficient areas. (Top) the covalent bond in the hydrogen molecule showing electron build up in the bonding region between two indivual hydrogen atoms.
CC BY-SA[5]
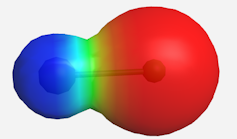 The ionic bond in sodium chloride (table salt) showing electron transfer to the chlorine side (right).
CC BY-SA[6]
The ionic bond in sodium chloride (table salt) showing electron transfer to the chlorine side (right).
CC BY-SA[6]
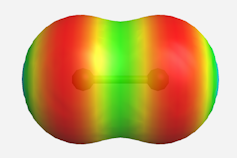 The charge-shift bond of the fluorine molecule showing electron depletion in the bonding region.
CC BY-SA[7]
What makes a charge-shift bond different?
In charge-shift bonds, electrons are both shared and transferred at the same time.
That might sound a little crazy, but think of it like this: You know those movable walkways at airports? Suppose that for over 100 years, people thought that the only way to get from one point to another was to either stand on the moving walkway or walk alongside it.
Now suppose that someone realized that there is a third way to move: You can stand on the walkway and walk at the same time. The speed at which you move through the airport is not due to standing or walking, but a combination of both.
Along with Shaik, Hiberty[8] and a handful of others around the world, I have helped[9] show that charge-shift bonding is a broad phenomenon that happens between a variety of elements from across the periodic table.
What inspired this discovery?
Shaik and Hiberty were calculating the energy required to break a series of bonds using a method called valence bond theory. Chemistry is all about pattern recognition, and all of the bonds they studied fit a well-established pattern, except the bond between two fluorine atoms. Traditionally thought of as a purely covalent bond, this molecule didn’t behave like any other covalent bond. By trying to understand why, Shaik and Hiberty uncovered something completely unique.
Why is it important?
This is the first major change in the way chemists think about bonding in more than 100 years. Chemical bonding is at the heart of chemistry, so changing the way chemists think about bonding will change the entire field.
How are charge-shift bonds applied in the real world?
Synthetic materials[10] such as computer chips[11], plastics[12], cosmetics[13], textiles[14] and medicines[15] come from making and breaking chemical bonds.
Therefore, insight into chemical bonding can inspire new materials with properties we have yet to imagine. We are already seeing chemists exploit the properties of charge-shift bonds to speed up chemical reactions and to understand the properties of industrial solvents.
What is the coolest element of your new research?
Chemistry is alive and constantly changing – that’s what first attracted me to the field. Charge-shift bonding challenges something so fundamental to the field that it is largely taken for granted.
The drama of sweeping theory change is in full effect here: The concept was introduced many years ago but not rapidly accepted; over time, diligent work by a handful of believers provided more support for the idea; and now it is gaining widespread acceptance[16] due to verification through alternative experimental[17] and theoretical[18] means.
I also find it fascinating that most chemical processes can now be reliably modeled on a computer. I always liked chemistry for the knowledge it provided about how things work on the atomic scale. However, I never felt comfortable playing with beakers and hazardous chemicals. While chemistry is still a predominantly experimental science, today computers can direct those experiments while also providing a place for an experimentally challenged chemist such as myself.
[Deep knowledge, daily. Sign up for The Conversation’s newsletter[19].]
The charge-shift bond of the fluorine molecule showing electron depletion in the bonding region.
CC BY-SA[7]
What makes a charge-shift bond different?
In charge-shift bonds, electrons are both shared and transferred at the same time.
That might sound a little crazy, but think of it like this: You know those movable walkways at airports? Suppose that for over 100 years, people thought that the only way to get from one point to another was to either stand on the moving walkway or walk alongside it.
Now suppose that someone realized that there is a third way to move: You can stand on the walkway and walk at the same time. The speed at which you move through the airport is not due to standing or walking, but a combination of both.
Along with Shaik, Hiberty[8] and a handful of others around the world, I have helped[9] show that charge-shift bonding is a broad phenomenon that happens between a variety of elements from across the periodic table.
What inspired this discovery?
Shaik and Hiberty were calculating the energy required to break a series of bonds using a method called valence bond theory. Chemistry is all about pattern recognition, and all of the bonds they studied fit a well-established pattern, except the bond between two fluorine atoms. Traditionally thought of as a purely covalent bond, this molecule didn’t behave like any other covalent bond. By trying to understand why, Shaik and Hiberty uncovered something completely unique.
Why is it important?
This is the first major change in the way chemists think about bonding in more than 100 years. Chemical bonding is at the heart of chemistry, so changing the way chemists think about bonding will change the entire field.
How are charge-shift bonds applied in the real world?
Synthetic materials[10] such as computer chips[11], plastics[12], cosmetics[13], textiles[14] and medicines[15] come from making and breaking chemical bonds.
Therefore, insight into chemical bonding can inspire new materials with properties we have yet to imagine. We are already seeing chemists exploit the properties of charge-shift bonds to speed up chemical reactions and to understand the properties of industrial solvents.
What is the coolest element of your new research?
Chemistry is alive and constantly changing – that’s what first attracted me to the field. Charge-shift bonding challenges something so fundamental to the field that it is largely taken for granted.
The drama of sweeping theory change is in full effect here: The concept was introduced many years ago but not rapidly accepted; over time, diligent work by a handful of believers provided more support for the idea; and now it is gaining widespread acceptance[16] due to verification through alternative experimental[17] and theoretical[18] means.
I also find it fascinating that most chemical processes can now be reliably modeled on a computer. I always liked chemistry for the knowledge it provided about how things work on the atomic scale. However, I never felt comfortable playing with beakers and hazardous chemicals. While chemistry is still a predominantly experimental science, today computers can direct those experiments while also providing a place for an experimentally challenged chemist such as myself.
[Deep knowledge, daily. Sign up for The Conversation’s newsletter[19].]
References
- ^ John Morrison Galbraith (www.researchgate.net)
- ^ Sason Shaik (yfaat.ch.huji.ac.il)
- ^ Philippe Hiberty (pagesperso.lcp.u-psud.fr)
- ^ the charge-shift bond (doi.org)
- ^ CC BY-SA (creativecommons.org)
- ^ CC BY-SA (creativecommons.org)
- ^ CC BY-SA (creativecommons.org)
- ^ Along with Shaik, Hiberty (doi.org)
- ^ have helped (doi.org)
- ^ materials (www.ted.com)
- ^ computer chips (cen.acs.org)
- ^ plastics (cen.acs.org)
- ^ cosmetics (cenblog.org)
- ^ textiles (cen.acs.org)
- ^ medicines (cen.acs.org)
- ^ widespread acceptance (www.chemistryworld.com)
- ^ experimental (doi.org)
- ^ theoretical (doi.org)
- ^ Sign up for The Conversation’s newsletter (theconversation.com)
Authors: John Morrison Galbraith, Associate Professor of Chemistry, Marist College
Read more https://theconversation.com/a-new-type-of-chemical-bond-the-charge-shift-bond-130087

