Why it's wrong to blame livestock farms for coronavirus
- Written by Alison Van Eenennaam, Researcher, Department of Animal Science, University of California, Davis
As part of the global response to the current pandemic, scientists are trying to identify the source of the SARS-CoV-2 virus. Some commentators have promoted unsubstantiated theories suggesting that “factory farming[1],” or intensive large-scale livestock agriculture[2] is to blame for this virus.
SARS-CoV-2 appears to have originated in bats[3], and possibly moved to humans via an intermediate host animal. Bats may also have been the original hosts[4] for outbreaks that infected humans via civets[5] in 2003 and camels[6] in 2012.
While these coronaviruses originated in animals, not one of these species is associated with “factory farming.” That makes it hard to follow the logic that farmed animals[7], rather than wildlife, resulted in the current pandemic. Animals, including pets[8], sometimes carry harmful germs that can spread to people. But studies show that the majority[9] of emerging zoonotic[10] diseases originate in wildlife.
And as an animal scientist[11], I have seen solid evidence[12] that biosecurity[13] practices associated with commercial animal agriculture decrease the risk of disease. While these practices – vaccination, physical distancing and preventing contact with wildlife - may not align with popular ideas of “naturalness,” they make livestock operations safer for both animals and humans.
Curbing outbreaks in livestock
Just as humans can experience pandemics, animal populations are also vulnerable to widespread disease events, known as panzootics. The World Organization of Animal Health[14] estimates that illness and death due to animal diseases cause annual losses of at least 20%[15] of livestock production globally.
The world’s 1 billion livestock keepers[16] have a strong incentive to control zoonotic diseases. These outbreaks threaten animal farmers’ health and economic well-being[17]. In general, large-scale intensive industries are able to implement biosecurity[18] more effectively than small-holder or extensive industries, where animals are outdoors.
Toward this end, multidisciplinary “One Health[19]” experts work in partnership on approaches to achieve the best health outcomes for people, animals and the environment. Commercial livestock farmers consult regularly with veterinarians and animal nutritionists, vaccinate against diseases, “socially distance” their young stock from each other and physically distance their herds and flocks from wildlife to reduce their exposure to natural reservoirs[20] of microbes. The risk of disease outbreaks increases when biosecurity protocols fail, or are absent.
Basic biosecurity for U.S. teens exhibiting animals at fairs.Cattle get pneumonia too
To see how livestock farmers work to curb infectious outbreaks, let’s consider viral respiratory diseases. These pathogens can cause pneumonia in cattle, just as SARS-CoV-2 does in people. Over 1.2 million cattle die each year[21] in the U.S. from bovine respiratory diseases – about 1% of the national herd. This disease is epizootic, meaning that it does not infect humans, and is associated with multiple viruses, including a bovine coronavirus.
Respiratory pathogens spread through close contact and aerosol transmission. This is one reason why commercial dairy farmers raise their calves in hutches that are spaced roughly 4 feet apart[22], to prevent nose-to-nose contact. Think of it as bovine social distancing.
 A dairy farm in California physically distances individual calves to prevent nose-to-nose contact.
Alison Van Eenennaam, CC BY-ND[23]
A dairy farm in California physically distances individual calves to prevent nose-to-nose contact.
Alison Van Eenennaam, CC BY-ND[23]
Animal welfare groups[24] have criticized this system, arguing that raising calves in group housing improves their social and feeding behavior[25], although few studies document long-term benefits[26].
On the other hand, commingling the animals this way poses a high risk of spreading germs[27]. Just as humans are staying apart now to reduce transmission of the novel coronavirus, housing calves apart sacrifices some social interaction to reduce disease risks.
Respiratory disease in chickens
Isolating farm animals from infectious diseases spread by wildlife is a major reason why poultry and pigs are housed indoors. Animals raised in the open are at greater risk[28] for predation and contracting infectious diseases from wildlife.
This was evident in the spring of 2018, when virulent Newcastle disease – a highly contagious viral respiratory infection – was reported in backyard chickens in Los Angeles. Since then, over 1.2 million birds[29] have been euthanized or died from the disease. The infection has since spread to over 470 flocks[30] in California, Utah and Arizona, most of them backyard flocks. Although vaccines are part of an effective biosecurity plan, less than 10%[31] of backyard poultry are vaccinated.
Cormorants, pigeons and doves can carry Newcastle disease, so the California Department of Food and Agriculture recommends housing poultry indoors to isolate them from wild birds. Commercial poultry farmers are now on high alert to try to protect their flocks. The biggest challenge in controlling the outbreak has been people disobeying a quarantine that prohibits moving birds out of infected counties.[32][33][34][35][36]
Global pig populations at risk
China has the world’s largest pig population and provides half of global pork production. Small-scale and backyard farms with minimal biosecurity produce more than 60% of Chinese pork[37]. Feeding pigs food waste, such as uncooked meat or food products – referred to as swill – is a high biosecurity risk[38], but is common practice on Chinese farms.
For many years China feared the introduction of a highly contagious and deadly tick-borne virus that causes African swine fever[39], or ASF. This deadly hemorrhagic disease, with mortality rates approaching 100%, was first detected in Kenya in the 1920s. Since there is no vaccine available, the only approach to control the disease is biosecurity. The virus moves between pigs, wild boar and soft ticks, but is harmless to humans.
A prophetic 2017 paper[40] warned that ASF could reach China due to factors including international travel and commerce, swill feeding practices and the presence of wild boar populations. The disease surfaced there in 2018, likely through contaminated feed[41], and has rapidly spread into every Chinese province.
Before the ASF panzootic, China’s pork output was almost five times larger than U.S. production. That output has dropped sharply[42], doubling the price of pork in China, where it is the nation’s most-consumed meat.
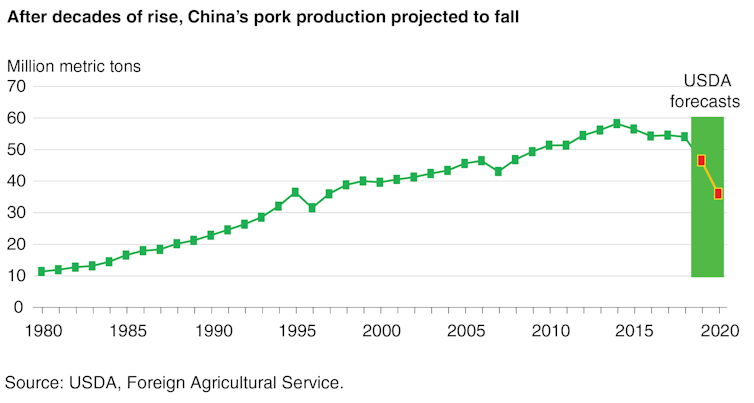 An outbreak of African swine fever, likely caused by contaminated feed, has sharply reduced pork production in China since 2018.
USDA[43]
An outbreak of African swine fever, likely caused by contaminated feed, has sharply reduced pork production in China since 2018.
USDA[43]
ASF has spread to much of Asia and still threatens pig populations globally[44]. Commercial pig producers in non-ASF countries have implemented strict biosecurity protocols, including managing feed sources[45], and customs agents are on high alert for smuggled pork products that could spread the disease further. Unfortunately, some travelers[46] seem oblivious to the potentially devastating impacts of dodging biosecurity measures.
Biosecurity works
Livestock farmers in developed nations understand the importance of biosecurity practices. That is why scientists believe the next important emerging pathogen is least likely[47] to originate from livestock in these countries.
In my view, the focus should be on controlling and eradicating emerging animal diseases[48] in resource-poor countries. Without such effort, more severe epizootic and zoonotic disease outbreaks are inevitable, threatening global health and food security.
[Get facts about coronavirus and the latest research. Sign up for The Conversation’s newsletter.[49]]
References
- ^ factory farming (www.theguardian.com)
- ^ livestock agriculture (www.theguardian.com)
- ^ originated in bats (doi.org)
- ^ may also have been the original hosts (dx.doi.org)
- ^ civets (dx.doi.org)
- ^ camels (dx.doi.org)
- ^ farmed animals (thehill.com)
- ^ pets (www.cdc.gov)
- ^ majority (doi.org)
- ^ zoonotic (www.cdc.gov)
- ^ animal scientist (scholar.google.com)
- ^ solid evidence (doi.org)
- ^ biosecurity (www.aphis.usda.gov)
- ^ World Organization of Animal Health (www.oie.int)
- ^ least 20% (www.icrad.eu)
- ^ 1 billion livestock keepers (doi.org)
- ^ economic well-being (cgspace.cgiar.org)
- ^ biosecurity (www.sciencedirect.com)
- ^ One Health (www.cdc.gov)
- ^ natural reservoirs (www.ncbi.nlm.nih.gov)
- ^ Over 1.2 million cattle die each year (dx.doi.org)
- ^ spaced roughly 4 feet apart (dx.doi.org)
- ^ CC BY-ND (creativecommons.org)
- ^ Animal welfare groups (www.theguardian.com)
- ^ improves their social and feeding behavior (doi.org)
- ^ long-term benefits (doi.org)
- ^ high risk of spreading germs (dx.doi.org)
- ^ greater risk (doi.org)
- ^ over 1.2 million birds (www.latimes.com)
- ^ 470 flocks (www.aphis.usda.gov)
- ^ less than 10% (dx.doi.org)
- ^ carry Newcastle disease (dx.doi.org)
- ^ housing poultry indoors (www.cdfa.ca.gov)
- ^ biggest challenge (www.avma.org)
- ^ people disobeying a quarantine (www.latimes.com)
- ^ moving birds out of infected counties. (www.cdfa.ca.gov)
- ^ more than 60% of Chinese pork (dx.doi.org)
- ^ high biosecurity risk (doi.org)
- ^ African swine fever (dx.doi.org)
- ^ 2017 paper (dx.doi.org)
- ^ likely through contaminated feed (dx.doi.org)
- ^ dropped sharply (www.ers.usda.gov)
- ^ USDA (www.ers.usda.gov)
- ^ globally (www.fao.org)
- ^ managing feed sources (dx.doi.org)
- ^ some travelers (www.reuters.com)
- ^ least likely (doi.org)
- ^ controlling and eradicating emerging animal diseases (www.oie.int)
- ^ Sign up for The Conversation’s newsletter. (theconversation.com)
Authors: Alison Van Eenennaam, Researcher, Department of Animal Science, University of California, Davis
Read more https://theconversation.com/why-its-wrong-to-blame-livestock-farms-for-coronavirus-137055

