Hunting for rare isotopes: The mysterious radioactive atomic nuclei that will be in tomorrow's technology
- Written by Artemis Spyrou, Associate Professor of Nuclear Physics, Michigan State University
When you hear the term “radioactive” you likely think “bad news,” maybe along the lines of fallout from an atomic bomb.
But radioactive materials are actually used in a wide range of beneficial applications. In medicine, they routinely help diagnose and treat disease. Irradiation helps keep a number of foods free from insects and invasive pests. Archaeologists use them to figure out how old an artifact might be. And the list goes on.
So what is radioactivity?
It’s the spontaneous emission of radiation when an atom’s dense center – called its nucleus – transforms into a different one. Whether in the form of particles or electromagnetic waves called gamma rays, radiation transfers energy away from the atomic nucleus.
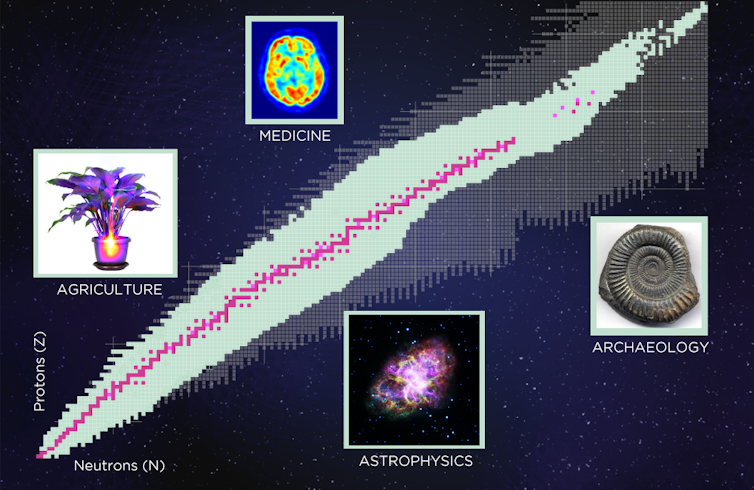 The nuclear chart showing the 250 or so stable isotopes in pink, the around 3,000 known rare isotopes in green and the approximately 4,000 predicted isotopes in grey.
Erin O'Donnell, Michigan State University, CC BY-ND[1]
The nuclear chart showing the 250 or so stable isotopes in pink, the around 3,000 known rare isotopes in green and the approximately 4,000 predicted isotopes in grey.
Erin O'Donnell, Michigan State University, CC BY-ND[1]
Through experiments, nuclear physicists have seen about 3,000 different kinds of nuclei to date. Current theories, though, predict the existence of about 4,000 more that have never yet been observed. Around the world, thousands of scientists, including me[2], continue to study these tiny constituents of matter, while governments spend billions of dollars on building powerful new machines that will produce more and more exotic nuclei – and maybe eventually more technologies that will further improve modern life.
The birth of nuclear physics
 Henri Becquerel, 1904.
Library of Congress[3]
Henri Becquerel, 1904.
Library of Congress[3]
French physicist Henri Becquerel[4] discovered natural radioactivity in 1896. He was trying to study how uranium salts phosphoresce – that is, emit light – when they’re exposed to sunlight. Becquerel placed a uranium sample on a photographic plate covered with opaque paper and left it in direct sunlight. The plate got foggy, which he concluded was due to sun exposure.
Thanks to a few days of cloudy weather, though, Becquerel left his whole setup in a dark drawer. Surprisingly, the photographic plate still fogged up, even in the absence of light. Sunlight had nothing to do with his previous observation. It was the natural radioactivity of the uranium samples that had this effect. As the uranium nuclei decayed – that is, transformed into different nuclei – they spontaneously emitted lightwaves that registered on the photographic plates.
Becquerel’s discovery ushered in a new era of physics and launched the field of nuclear science. For this work, he won the Nobel Prize in 1903.
Since then, nuclear scientists have unraveled a lot of the inner workings of the atomic nucleus, and have harnessed its amazing energy both for good and unfortunately not so good uses. Nuclear physics discoveries have given us ways to look inside our bodies noninvasively, ways to create energy without air pollution, and ways to study our history and our environment.
On the atomic level
The known atomic nuclei belong to 118 different elements, some of them naturally occurring and some of them human-made. For every element on the periodic table there are many different “isotopes,” from the Greek word “ισότοπο,” which means “same place,” implying the same place on the periodic table of the elements.
To be the same element, two isotopes must have the same number of protons – the positively charged subatomic particle. It’s their number of neutrons – subatomic particles with no charge at all – that can vary significantly.
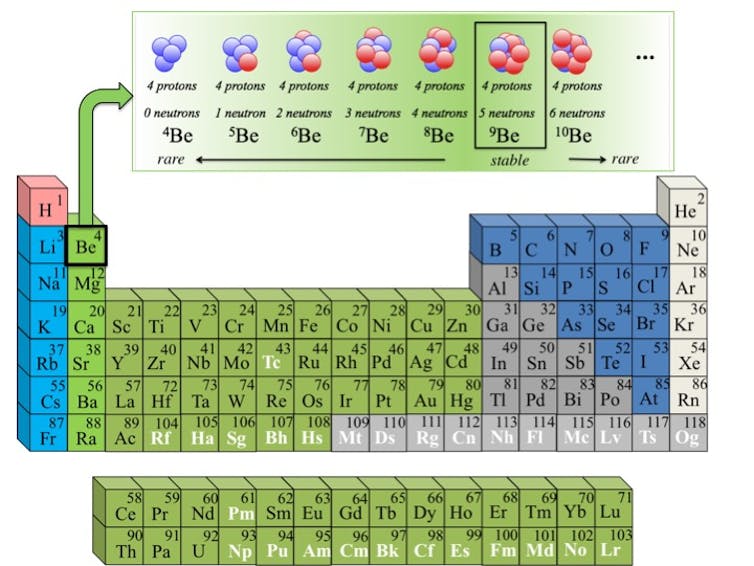 The periodic table lists all the elements based on their number of protons. Isotopes of an element have the same number of protons – for Beryllium it’s four – but various numbers of neutrons.
Artemis Spyrou, CC BY-ND[5]
The periodic table lists all the elements based on their number of protons. Isotopes of an element have the same number of protons – for Beryllium it’s four – but various numbers of neutrons.
Artemis Spyrou, CC BY-ND[5]
For example, gold is element 79 on the periodic table, and all isotopes of gold will have the same metallic, yellowish appearance. However, there are 40 known isotopes of gold that have been discovered, and another roughly 20 are theorized to exist. Only one of these isotopes is the “stable,” or naturally occurring, form of gold you might be wearing on your ring finger right now. The rest are radioactive isotopes, also known as “rare isotopes.”
Rare isotopes each have unique properties: They live for different amounts of time, from a fraction of a second to a few billion years, and they release different types of radiation and different amounts of energy.
For example, modern smoke detectors use the isotope Americium-241[6], which emits a type of radiation called alpha particles that have a very short range. The radioactivity can’t travel more than a couple of inches in air. Americium-241 lives for a few hundred years.
On the other hand, the isotope Fluorine-18, which is commonly used in medical PET scans, lives for only about 100 minutes – long enough to complete the scan, but short enough to avoid irradiating the healthy body unnecessarily for an extended period. The secondary electromagnetic radiation that comes from Fluorine-18 is in the form of long-range gamma rays, which allows it to travel out of the body and into the PET cameras.
These different nuclear properties make each rare isotope unique, and nuclear physicists have to design specialized experiments to study each one of them separately.
Hunting for more
Current nuclear science research strives to develop new techniques for discovering new isotopes, understanding their properties, and eventually producing and harvesting them efficiently.
Producing rare isotopes is not an easy task[7]; it requires large machines that will make nuclei travel, and collide with each other, at speeds close to the speed of light. During these collisions nuclei can fuse together, or they can break each other apart, producing new nuclei, potentially with previously unseen combinations of protons and neutrons.
Nuclear physicists have dedicated equipment - detectors - that can observe these newly formed nuclei and the radiation they emit, and study their properties. For example, at the National Superconducting Cyclotron Laboratory[8] where I work[9], my group has developed an extremely efficient gamma ray detector we called SuN.
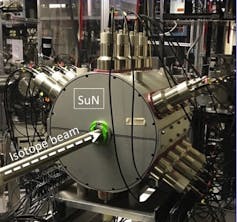 The SuN detector at the National Superconducting Cyclotron Laboratory measures gamma rays and helps researchers study the properties of rare isotopes.
Artemis Spyrou, CC BY-ND[10]
The SuN detector at the National Superconducting Cyclotron Laboratory measures gamma rays and helps researchers study the properties of rare isotopes.
Artemis Spyrou, CC BY-ND[10]
The majority of the known isotopes emit gamma radiation when they decay. We want to know how much energy is released in this process, how many different gamma rays are emitted and how the energy is shared between them, and how long it takes for the decay to take place. SuN can answer these questions about whichever isotope we are investigating.
In a typical experiment, we implant a beam of rare isotopes at the center of SuN. The rare isotopes will decay of their own accord after a short amount of time, roughly one second or less, and emit their characteristic radiation. SuN detects these emitted gamma rays. It’s our job as nuclear experimentalists to put together the puzzle of how those gamma rays were emitted and what they tell us about the properties of the new isotope.
These kinds of production and detection techniques are complex and costly, and therefore there are only a handful of rare isotope laboratories in the world that can produce and study the most exotic nuclear species.
It’s impossible to predict which new discoveries in basic research will have an impact on people’s lives. Who could have known 100 years ago, when the electron was discovered, that for a few decades almost every house in the developed world would have an electron machine – otherwise known as a cathode-ray tube[11] – to watch television? And who could have guessed that the discovery of radioactivity would eventually lead to space exploration powered by radioactive decays[12]?
In the same way, we cannot predict which rare isotope discoveries will be the game-changers, but with more than half of the predicted isotopes still unexplored, to me the possibilities feel endless.
References
- ^ CC BY-ND (creativecommons.org)
- ^ including me (www.artemisspyrou.com)
- ^ Library of Congress (commons.wikimedia.org)
- ^ Henri Becquerel (www.nobelprize.org)
- ^ CC BY-ND (creativecommons.org)
- ^ use the isotope Americium-241 (www3.epa.gov)
- ^ is not an easy task (www.youtube.com)
- ^ National Superconducting Cyclotron Laboratory (www.nscl.msu.edu)
- ^ where I work (scholar.google.com)
- ^ CC BY-ND (creativecommons.org)
- ^ cathode-ray tube (electronics.howstuffworks.com)
- ^ space exploration powered by radioactive decays (rps.nasa.gov)
Authors: Artemis Spyrou, Associate Professor of Nuclear Physics, Michigan State University

