A day to celebrate chemistry’s favorite unit — the mole. But what’s a mole?
- Written by Tara S. Carpenter, Senior Lecturer and General Chemistry Coordinator, University of Maryland, Baltimore County
On Oct. 23, between 6:02 a.m. and 6:02 p.m., chemists celebrate Mole Day. Mole Day is not a day to celebrate those furry little creatures that live in the ground. Rather, it is a day to celebrate a very important idea in the sub-microscopic world.
In chemistry, the mole is a unit used to talk about atoms. It is similar to other units we use everyday. For example, you might walk into the local donut shop and order a dozen donuts. In doing so, you know that you will get 12 donuts and the clerk knows to give you 12 donuts. The dozen unit is simply for convenience in discussing a quantity.
We apply the same idea to discuss quantities of atoms. Why do we not simply talk about dozens of atoms? The reason is because atoms are so small that it doesn’t make sense to do so. Imagine a single grain of table salt. That tiny crystal contains over 1,000,000,000,000,000,000 (one quintillion) atoms. Rather than discussing such a large number of atoms, we can talk more conveniently through the mole unit. A mole of something contains 602,000,000,000,000,000,000,000 or 6.02 x 10²³ of that thing.
That number is also called Avogadro’s number. Amedeo Avogadro was an Italian physicist. In 1811, he proposed that equal volumes of any gas at the same temperature and pressure contain the same number of atoms (or molecules). The number is named after him to honor his work. Because Oct. 23 is abbreviated as 10/23, chemists use this date to celebrate Mole Day. Instead of talking about over 1,000,000,000,000,000,000 atoms in the grain of salt, we can express the quantity as around 0.000002 moles of atoms, which is much more convenient.
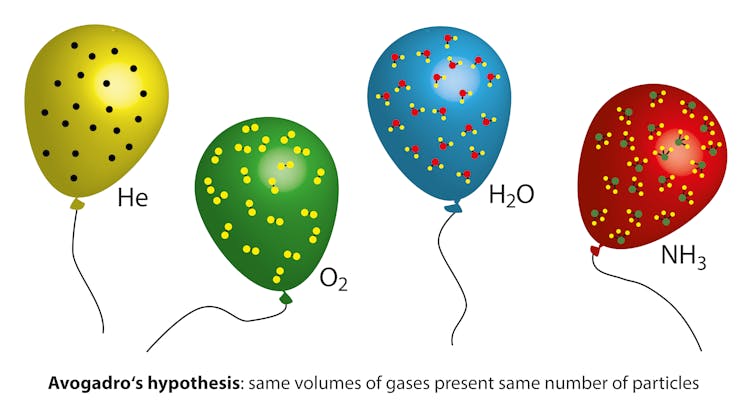 Amedeo Avogadro predicted that equal volumes of different gases would contain the same number of molecules.
magnetix / Shuterstock.com[1]
Amedeo Avogadro predicted that equal volumes of different gases would contain the same number of molecules.
magnetix / Shuterstock.com[1]
How much space does a mole occupy?
Now just how many is 6.02 x 10²³? How long do you think it would take you to count to a mole? One day? One week? One year? Go ahead, start counting. It would take you around 20,000,000,000,000,000 years. As you can see, very large quantities of atoms take up very little space which gives us an idea of just how tiny they are. Here is another example: One mole of water with all 6.02 x 10²³ molecules of H₂O occupies slightly more than a tablespoon.
So how do those tiny atoms come together to make up the stuff in the world around us? Even though atoms are so small, there is a lot of action going on. Each atom is made up of even smaller particles called electrons. The way those electrons place themselves around the atom lead to properties we can experience and observe. In a metal, the tiny atoms are swimming in a sea of electrons which gives them the ability to conduct heat and electricity.
How about water? The electrons in a molecule of water are arranged so that each water molecule is extremely attracted to the one next to it. Because of this they naturally arrange themselves at the atomic level in ways that have big consequences in the world around us. When water freezes, the molecules arrange in a way that creates a lattice that causes ice to float in liquid water. Why is that so important? Because ice floats, a pond or lake will freeze at the top, but below the entire aquatic ecosystem is able to survive. This is an amazing phenomenon of water.
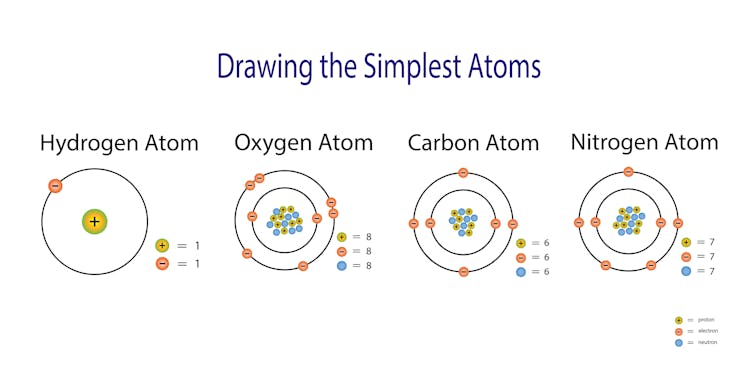 Atoms are made up of even smaller particles called protons, neutrons and electrons. The arrangement of these particles gives each substance specific properties.
Nasky / Shutterstock.com[2]
Atoms are made up of even smaller particles called protons, neutrons and electrons. The arrangement of these particles gives each substance specific properties.
Nasky / Shutterstock.com[2]
Small atoms with big consequences
Many other substances adopt their own unique properties due to arrangement of electrons. The propane gas that we use to fuel a gas grill is a gas at room temperature because the molecules are weakly attracted to each other. Unlike water, they don’t really want to be next to each other at all. Consequently, the space between them results in a gaseous state.
Another important gas is oxygen. We need oxygen to live out our lives. Close your eyes and take a deep breath. As you do that, the molecules are whizzing through your nose, into your lungs where about 0.001 moles of oxygen are absorbed into your blood. Those molecules are responsible for helping each cell in your body produce energy so that your eyes can see the words on this page and your brain can think about what they mean, all while keeping your heart beating.
So, if you ever feel like you’re too insignificant to make a difference, just remember that even the smallest of things matter in the grand scheme of things.
Happy Mole Day!
References
- ^ magnetix / Shuterstock.com (www.shutterstock.com)
- ^ Nasky / Shutterstock.com (www.shutterstock.com)
Authors: Tara S. Carpenter, Senior Lecturer and General Chemistry Coordinator, University of Maryland, Baltimore County

