Starting with Mother Nature's designs will speed up critical development of new antibiotics
- Written by Natalie Jones Slivinski, Virology Research Scientist, University of Washington
“I did not invent penicillin. Nature did that. I only discovered it by accident.” - Alexander Fleming
Natural products have been the basis of medicine for centuries. Aspirin is based on a chemical in willow tree bark[1]. Morphine comes from the opium plant[2]. Penicillin was discovered in a mold[3].
Natural products used in drug therapies are complex, diverse, highly specialized compounds produced by living things. Many evolved as defense mechanisms against other organisms. Certain microbes, for example, spew out potent antibacterial toxins that kill competing microbe species. Streptomycin, chloramphenicol and tetracycline – three of the most widely used antibiotics – were all discovered in soil bacteria[4]. Nature is the grand architect behind a major[5] proportion[6] of modern drugs.
At a time when antibiotic-resistant infections are running rampant, the need for effective new drugs is acute. Every year, drug-resistant bacteria cause[7] over 2 million infections and 23,000 deaths in the United States alone. And yet, despite their effectiveness, pharmaceutical companies often overlook natural compounds, instead focusing on subpar synthetic ones. Using current technologies to revisit natural products could help researchers identify badly needed new drugs, particularly antibiotics.
 Some of Alexander Fleming’s original penicillin mold.
AP Photo/Alastair Grant[8]
Some of Alexander Fleming’s original penicillin mold.
AP Photo/Alastair Grant[8]
The first ‘golden age’ of antibiotics
Fleming’s discovery of penicillin in 1929 launched the antibiotic “golden age[9].” In the years surrounding World War II, the pharmaceutical industry churned out dozens of new antibiotics in over 20 unique categories[10]. A few were engineered in the lab, but most were discovered in microbes. These new drugs led to a dramatic decrease in bacterial infections worldwide, increasing the average life expectancy by several[11] decades[12]. Things were looking good.
Sadly, it couldn’t last. In the 1960s, the discovery of new antibiotic categories, or classes, came to a screeching halt. Since then, only two new classes[13] have come to market. After years of blasting infections with the same classes of antibiotics, organisms had evolved resistance mechanisms; many existing antibiotics stopped working. Having picked the low-hanging fruit of antibacterials, our arsenal was drying up. Bacteria are developing resistance faster than we’re coming up with new weapons.
Rise of high-throughput screening
Researchers in the 1980s[14] started to focus on a rising technology called high-throughput screening (HTS). Automated systems test thousands – even millions – of compounds per year. The goal is to identify compounds that would spell bad news for infectious agents. Researchers observe how effective each compound is against a potential target – for example, in disrupting the bacterial cell wall or hindering its ability to synthesize DNA, RNA or protein. Many HTS systems aim for just one of these processes at a time in a plastic multi-well plate.
Some top-of-the-line HTS robots can push through 100,000 compounds per day[15]. The idea is that by screening millions of compounds, researchers are bound to find some with antimicrobial activity.
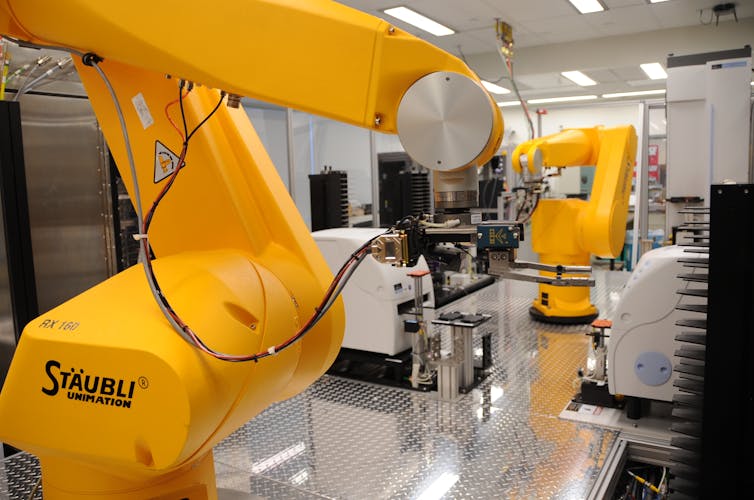 Automation lets robots screen massive amounts of compounds very quickly.
Maggie Bartlett, National Human Genome Research Institute[16]
Automation lets robots screen massive amounts of compounds very quickly.
Maggie Bartlett, National Human Genome Research Institute[16]
To save costs, pharmaceutical companies put together compound libraries: huge databases of small molecules in just about every configuration they could think of. Despite their proven track record, many companies decided natural products had low economic value[17] and instead turned to cheaper synthetic chemicals. These are screened against pathogen targets in the search for “hits”: cases where the database molecule affects the infectious agent.
Prioritizing quantity over quality
It turns out, scientists aren’t as good at designing antibiotics as we’d hoped. Compared to natural products, synthetic compounds simply haven’t been a high-quality source of drugs. Even after years of fine-tuning HTS, success rates for novel compounds are extremely low[18]. Pharmaceutical companies might spend years looking for drug candidates and still come up empty.
In fact, natural products still account for half[19] of newly discovered drugs since the 1980s, and approval rates for naturally derived products are climbing[20], even though very few are screened compared to synthetic compounds.
Time and money are precious resources in drug development; it takes 10 to 15 years and millions of dollars[21] – even[22] billions[23] – to develop a single drug from “farm-to-table.” There are four major steps of drug development:
- Screen compound library and identify “hits”;
- Confirm hits with further testing, at which point they become “lead compounds”;
- Advance leads through clinical trials[24];
- And finally, successful release of the drug.
From beginning to end, maybe 1 in 10 million[25] compounds screened[26] – and that’s a generous estimate – will become a successful drug for infectious diseases[27]. This number has not significantly improved over the years.
 A robot plate carousel could hold natural compounds just as easily as synthesized ones.
Daniel Soñé Photography, LLC, CC BY[28][29]
A robot plate carousel could hold natural compounds just as easily as synthesized ones.
Daniel Soñé Photography, LLC, CC BY[28][29]
Bring back the old-school methods
One reason natural products are such a promising resource for new drugs is that they are more biologically relevant[30] than synthetics; they’re ready-made to be active within cells. They contain fewer heavy metals and can be extremely stable[31]. Most importantly, because of their high complexity and diversity, a single natural compound often simultaneously targets multiple bacterial processes (for instance, both the cell wall and protein synthesis), making it less susceptible to resistance[32].
In comparison, high-throughput screening usually involves pinpointing only single targets – for instance, a particular bacterial enzyme or viral protein. Then, follow-up experiments will determine whether the drug-target interaction actually works within a cell, and not just in a test tube. This is incredibly inefficient and is a crucial limitation of classic HTS.
The most effective antibiotics are discovered by testing for antibacterial, antifungal or antiviral activity first, and then teasing apart the molecular mechanism. This means turning the focus back to bacterial assays, where compounds are tested in live bacterial cell cultures from the start. Newer HTS systems do target whole-cell systems, but much of the pharmaceutical industry still persists in using synthetic small-molecule libraries and shies away from naturally derived products.
This doesn’t mean that HTS has no place in drug development. But a meal is only as good as its ingredients, and high throughput is useless without high-quality compounds.
There are certainly barriers to natural product research. When it comes to plant-based chemicals, for example, high throughput can be a challenge[33]; purifying a specific chemical from pulverized plant material can be difficult to do the exact same way every time. Natural products are also difficult to patent, so for pharmaceutical companies, large compound libraries are more economically[34] viable[35].
However, with improved technology, HTS of natural products is becoming a reality, particularly when it comes to compounds produced by microbes[36]. Mass production is also getting easier; not just for bacteria, which are straightforward to culture on a large scale, but for plant-based chemicals too. In 2006, for example, researchers at UC Berkeley[37] found a way to engineer yeast to mass-produce the precursor for artemisinin, the antimalarial and anti-TB[38] compound that comes from the herb Artemisia annua.
 Plants and microbes likely hold many unknown but useful compounds.
Trisha, CC BY-ND[39][40]
Plants and microbes likely hold many unknown but useful compounds.
Trisha, CC BY-ND[39][40]
Potential gold mines for natural compounds
There is a huge area of untapped resources for natural anti-pathogenic molecules. A very small portion – less than 15 percent[41] – of terrestrial plants have been explored for natural product research; strikingly, less than 1 percent[42] of the microbial world has been tapped.
Almost two-thirds of natural products[43] come from a group of bacteria called actinomycetes, which include the streptomycetes. They produce antibacterial, antifungal, antiparasitic, immunosuppressant and anti-viral compounds. Potent anti-viral compounds have been found in fungi, plants and even marine sponges[44]. Drugs could potentially even come from the microbiome in our own gut[45].
Currently, I study certain plant-based extracts, traditionally used as anti-inflammatories in non-Western medicine, that actually have antiviral properties. Feverfew[46], for example, can protect cells against viruses like herpes simplex[47] and Epstein-Barr[48] by inhibiting inflammatory pathways. Artemisinin, mentioned earlier as an antimalaria drug, also has broad-spectrum activity against many different viruses[49].
Drug-resistant infections are a major global health threat[50]. It is critical that drug developers push for high-quality source material in their search for new drugs. It’s time to use technology to revive and upgrade tried-and-true methods.
References
- ^ willow tree bark (www.pharmaceutical-journal.com)
- ^ opium plant (www.ncbi.nlm.nih.gov)
- ^ in a mold (doi.org)
- ^ soil bacteria (doi.org)
- ^ major (doi.org)
- ^ proportion (doi.org)
- ^ drug-resistant bacteria cause (www.cdc.gov)
- ^ AP Photo/Alastair Grant (www.apimages.com)
- ^ golden age (dx.doi.org)
- ^ 20 unique categories (doi.org)
- ^ several (www.ncbi.nlm.nih.gov)
- ^ decades (www.ncbi.nlm.nih.gov)
- ^ only two new classes (doi.org)
- ^ in the 1980s (doi.org)
- ^ 100,000 compounds per day (academic.oup.com)
- ^ Maggie Bartlett, National Human Genome Research Institute (commons.wikimedia.org)
- ^ low economic value (dx.doi.org)
- ^ extremely low (doi.org)
- ^ account for half (doi.org)
- ^ are climbing (doi.org)
- ^ millions of dollars (www.sciencemag.org)
- ^ even (doi.org)
- ^ billions (doi.org)
- ^ through clinical trials (www.fda.gov)
- ^ 1 in 10 million (www.ncbi.nlm.nih.gov)
- ^ compounds screened (doi.org)
- ^ successful drug for infectious diseases (www.bio.org)
- ^ Daniel Soñé Photography, LLC (www.flickr.com)
- ^ CC BY (creativecommons.org)
- ^ more biologically relevant (doi.org)
- ^ extremely stable (dx.doi.org)
- ^ less susceptible to resistance (doi.org)
- ^ can be a challenge (doi.org)
- ^ economically (doi.org)
- ^ viable (doi.org)
- ^ produced by microbes (doi.org)
- ^ researchers at UC Berkeley (doi.org)
- ^ anti-TB (www.scidev.net)
- ^ Trisha (www.flickr.com)
- ^ CC BY-ND (creativecommons.org)
- ^ less than 15 percent (doi.org)
- ^ less than 1 percent (doi.org)
- ^ two-thirds of natural products (doi.org)
- ^ marine sponges (pubs.rsc.org)
- ^ the microbiome in our own gut (doi.org)
- ^ Feverfew (doi.org)
- ^ herpes simplex (doi.org)
- ^ Epstein-Barr (doi.org)
- ^ many different viruses (doi.org)
- ^ global health threat (www.who.int)
Authors: Natalie Jones Slivinski, Virology Research Scientist, University of Washington

