Graphene is a proven supermaterial, but manufacturing the versatile form of carbon at usable scales remains a challenge
- Written by Kevin Wyss, PhD Student in Chemistry, Rice University
“Future chips may be 10 times faster, all thanks to graphene[1]”; “Graphene may be used in COVID-19 detection[2]”; and “Graphene allows batteries to charge 5x faster[3]” – those are just a handful of recent dramatic headlines lauding the possibilities of graphene. Graphene is an incredibly light, strong and durable material made of a single layer of carbon atoms. With these properties, it is no wonder researchers have been studying ways that graphene could advance material science and technology for decades.
I never know what to expect when I tell people I study graphene[4] – some have never heard of it, while others have seen some version of these headlines and inevitably ask, “So what’s the holdup?”
Graphene is a fascinating material, just as the sensational headlines suggest, but it is only just starting be used in real-world applications. The problem lies not in graphene’s properties, but in the fact that it is still incredibly difficult and expensive to manufacture at commercial scales.
What is graphene?
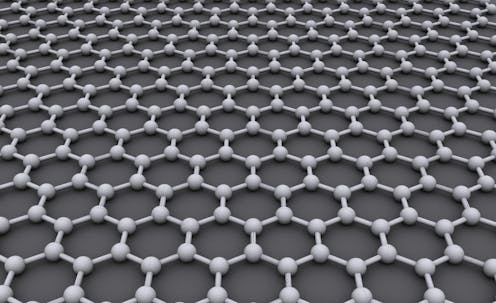
Graphene is most simply defined as a single layer of carbon atoms bonded together in a hexagonal, sheetlike structure. You can think of pure graphene as a one-layer-thick sheet of carbon tissue paper that happens to be the strongest material on Earth.
Graphene usually comes in the form of a powder made of small, individual sheets that are roughly the diameter of a grain of sand. An individual sheet of graphene is 200 times stronger than an equally thin piece of steel[7]. Graphene is also extremely conductive[8], holds together at up to 1,300 degrees Fahrenheit (700 C)[9], can withstand acids[10] and is flexible and very lightweight[11].
Because of these properties, graphene could be extremely useful. The material can be used to create flexible electronics[12] and to purify or desalinate water[13]. And adding just 0.03 ounces (1 gram) of graphene to 11.5 pounds (5 kilograms) of cement increases the strength of the cement by 35%[14].
As of late 2022, Ford Motor Co., with which I worked as part of my doctoral research, is one of the the only companies to use graphene at industrial scales. Starting in 2018, Ford began making plastic for its vehicles that was 0.5% graphene – increasing the plastic’s strength by 20%[15].
How to make a supermaterial
Graphene is produced in two principal ways that can be described as either a top-down or bottom-up process.
The world’s first sheet of graphene[17] was created in 2004 out of graphite. Graphite, commonly known as pencil lead, is composed of millions of graphene sheets stacked on top of one another. Top-down synthesis, also known as graphene exfoliation[18], works by peeling off the thinnest possible layers of carbon from graphite. Some of the earliest graphene sheets were made by using cellophane tape to peel off layers of carbon from a larger piece of graphite[19].
The problem is that the molecular forces holding graphene sheets together in graphite are very strong, and it’s hard to pull sheets apart. Because of this, graphene produced using top-down methods is often many layers thick, has holes or deformations, and can contain impurities[20]. Factories can produce a few tons of mechanically or chemically exfoliated graphene per year, and for many applications – like mixing it into plastic – the lower-quality graphene works well[21].
Top-down, exfoliated graphene is far from perfect, and some applications do need that pristine single sheet of carbon.
Bottom-up synthesis builds the carbon sheets one atom at a time over a few hours. This process – called vapor deposition[24] – allows researchers to produce high-quality graphene that is one atom thick and up to 30 inches across. This yields graphene with the best possible mechanical and electrical properties. The problem is that with a bottom-up synthesis, it can take hours to make even 0.00001 gram[25] – not nearly fast enough for any large scale uses like in flexible touch-screen electronics or solar panels[26], for example.
So what’s the holdup?
Current production methods of graphene, both top-down and bottom-up, are expensive as well as energy and resource intensive, and simply produce too little product, too slowly.
Some companies do manufacture graphene and sell it for US$60,000 to $200,000 per ton[27]. There are a limited number of uses that make sense at these high costs.
While small amounts of top-down or bottom-up graphene can satisfy the needs of researchers, for companies even just the process of prototyping a new material, application or manufacturing process requires many pounds of graphene powder or hundreds of graphene sheets and a lot of time and effort. It took significant investment and more than four years of study, development and optimization before graphene hit the production line at Ford.
Current production can barely cover experimentation, much less widespread use.
Improving manufacturing
For a material that has been around since only 2004, a lot of progress has been made in scaling up the production and implementation of graphene.
There are hints that graphene is starting to break through at a commercial level. There are a huge number of graphene-related startups looking at a wide range of uses[28] ranging from energy storage[29] to composites[30] to nerve stimulation[31]. Major companies – such as Tesla[32], LG and chemical giant BASF[33] – are also investigating how graphene could be used, in rechargeable batteries, flexible or wearable electronics and next-generation materials.
Graphene is ripe for a breakthrough that will bring down the cost and increase the scale of production, and this is an area of intense academic research[34]. One new technique discovered in 2020, called flash joule heating[35], is especially promising. Researchers have shown that passing large amounts of electricity through any carbon source reorganizes the carbon-carbon bonds into a graphene structure. Using this process, it is possible to make many pounds of high-quality graphene for a relatively low cost out of any carbon-containing material like coal or even trash. A company called Universal Matter Inc.[36] is already commercializing the process.
Once the cost of graphene comes down, the commercial applications will follow. The appetite for graphene is huge[37], but it is going to take some time before this material lives up to its potential.
References
- ^ 10 times faster, all thanks to graphene (www.msn.com)
- ^ used in COVID-19 detection (www.newswise.com)
- ^ charge 5x faster (www.theverge.com)
- ^ I study graphene (scholar.google.com)
- ^ M.H. Gass/Wikimedia Commons (commons.wikimedia.org)
- ^ CC BY (creativecommons.org)
- ^ 200 times stronger than an equally thin piece of steel (doi.org)
- ^ extremely conductive (doi.org)
- ^ up to 1,300 degrees Fahrenheit (700 C) (doi.org)
- ^ withstand acids (doi.org)
- ^ flexible and very lightweight (news.mit.edu)
- ^ create flexible electronics (www.azonano.com)
- ^ purify or desalinate water (www.azocleantech.com)
- ^ increases the strength of the cement by 35% (firstgraphene.net)
- ^ increasing the plastic’s strength by 20% (media.ford.com)
- ^ Rapid Eye/E+ via Getty Images (www.gettyimages.com)
- ^ first sheet of graphene (www.graphene-info.com)
- ^ graphene exfoliation (www.azonano.com)
- ^ peel off layers of carbon from a larger piece of graphite (science.wonderhowto.com)
- ^ can contain impurities (doi.org)
- ^ lower-quality graphene works well (www.compositesworld.com)
- ^ Дагесян Саркис Арменакович/Wikimedia Commons (commons.wikimedia.org)
- ^ CC BY-SA (creativecommons.org)
- ^ vapor deposition (www.graphenea.com)
- ^ hours to make even 0.00001 gram (doi.org)
- ^ flexible touch-screen electronics or solar panels (doi.org)
- ^ US$60,000 to $200,000 per ton (bigthink.com)
- ^ graphene-related startups looking at a wide range of uses (fortune.com)
- ^ energy storage (nanotechenergy.com)
- ^ composites (graphmatech.com)
- ^ nerve stimulation (www.inbrain-neuroelectronics.com)
- ^ Tesla (electrek.co)
- ^ chemical giant BASF (www.thegraphenecouncil.org)
- ^ area of intense academic research (www.phdassistance.com)
- ^ flash joule heating (doi.org)
- ^ company called Universal Matter Inc. (www.universalmatter.com)
- ^ appetite for graphene is huge (www.fortunebusinessinsights.com)
Authors: Kevin Wyss, PhD Student in Chemistry, Rice University

