Newly discovered species of bacteria in the microbiome may be a culprit behind rheumatoid arthritis
- Written by Meagan Chriswell, MD/PhD Candidate in Immunology, University of Colorado Anschutz Medical Campus
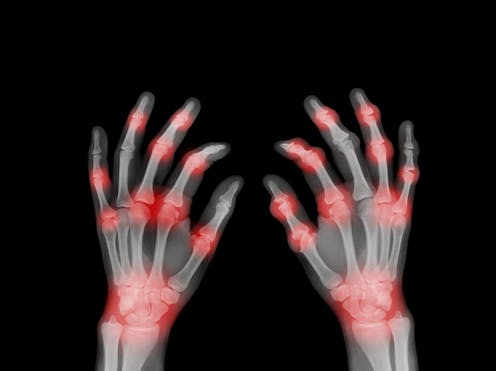
Rheumatoid arthritis affects 1 in 100 people worldwide[1]. It causes inflamed, painful and swollen joints, often in the hands and wrists, and can lead to loss of joint function as well as chronic pain and joint deformities and damage. What causes this condition has been unknown.
In our recently published study[2], my colleagues and I found an important clue to a potential culprit behind this disease: the bacteria in your gut.
What causes rheumatoid arthritis?
Rheumatoid arthritis is an autoimmune condition[3], meaning it develops when the body’s immune system starts to attack itself. Proteins called antibodies, which usually help fight off viruses and bacteria, begin to attack the joints instead.
The origins of the antibodies that cause rheumatoid arthritis have been an area of study for many years. Some research has shown[4] that these antibodies can start forming at sites like the mouth, lung and intestines over 10 years before symptoms arise. But until now, it was unclear why researchers were finding these antibodies in these particular areas.
Rheumatoid arthritis can develop at any age.We wanted to investigate what could trigger the formation of these antibodies. Specifically, we wondered if bacteria in the microbiome[5], a community of microorganisms that live in the intestines, might be the ones activating the immune response that leads to rheumatoid arthritis. Since microbes commonly live at the same sites as the antibodies driving rheumatoid arthritis, we hypothesized that these bacteria could be triggering the production of these antibodies. We reasoned that though these antibodies were meant to attack the bacteria, rheumatoid arthritis develops when they spread beyond the intestines to attack the joints.
First, we sought to identify the intestinal bacteria targeted by these antibodies. To do this, we exposed the bacteria in the feces of a subset of people at risk for developing rheumatoid arthritis to these antibodies, allowing us to isolate just the bacterial species that reacted and bound to the antibodies.
We found that one previously unknown species of bacteria was present in the intestines of around 20% of people who were either diagnosed with rheumatoid arthritis or produce the antibodies that cause the disease. As a member of the Cherokee Nation of Oklahoma, I suggested we name this species Subdoligranulum didolesgii (“didolesgii” means arthritis or rheumatism in Cherokee) as a nod to the contributions that other Indigenous scholars have made to science as well as the fact that rheumatoid arthritis affects Indigenous people[6] at a higher rate than other populations.
Subdoligranulum didolesgii has not been detected in the feces of healthy people before, and it is currently unknown how prevalent this bacteria is in the general population.
We also found that these bacteria can activate specialized immune cells called T cells in people with rheumatoid arthritis. T cells drive inflammatory responses in the body, and have been linked to the development of different autoimmune diseases[7].
These findings suggest that these gut bacteria may be activating the immune systems of people with rheumatoid arthritis. But instead of attacking the bacteria, their immune system attacks the joints.
Most of your immune system is located in your gut.Why this bacteria?
It is still unknown why people with rheumatoid arthritis develop an immune response to Subdoligranulum didolesgii. But we think it may be the culprit when it comes to rheumatoid arthritis because this bacteria is found only in the intestines of people with rheumatoid arthritis, and not in the intestines of healthy people.
While many immune responses happen in the intestines[8], they are usually self-contained and do not spread to other areas of the body. However, we believe that a particularly strong intestinal immune response against Subdoligranulum didolesgii could allow antibodies to bypass the intestinal “firewall” and spread to the joints.
To confirm our hypothesis, we gave mice an oral dose of Subdoligranulum didolesgii and monitored their reaction. Within 14 days, the mice began to develop joint swelling and antibodies that attacked their joints.
The future of rheumatoid arthritis treatment
My colleagues and I hope this research can shed light on the origins of rheumatoid arthritis. Our next goal is to discover how common these bacteria are in the general population and test whether the presence of these bacteria in the gut may lead to the development of rheumatoid arthritis in people.
It’s important to note that antibiotics[9] are unlikely to be helpful treatment for the microbiomes of patients with rheumatoid arthritis. Although Subdoligranulum didolesgii may be triggering an autoimmune response for some people with rheumatoid arthritis, antibiotics eliminate both helpful and harmful bacteria in the gut. Additionally, removing the bacteria won’t necessarily stop the immune system from attacking the joints once it has started.
Nevertheless, we believe that these bacteria can be used as tools to develop treatments for rheumatoid arthritis and hopefully ways to prevent disease from happening in the first place.
References
- ^ 1 in 100 people worldwide (rheumatoidarthritis.net)
- ^ recently published study (www.science.org)
- ^ autoimmune condition (www.niehs.nih.gov)
- ^ research has shown (doi.org)
- ^ microbiome (www.genome.gov)
- ^ affects Indigenous people (doi.org)
- ^ different autoimmune diseases (doi.org)
- ^ happen in the intestines (doi.org)
- ^ antibiotics (doi.org)
Authors: Meagan Chriswell, MD/PhD Candidate in Immunology, University of Colorado Anschutz Medical Campus

