Nobel Prize: How click chemistry and bioorthogonal chemistry are transforming the pharmaceutical and material industries
- Written by Heyang (Peter) Zhang, PhD Candidate in Chemistry, University at Buffalo
The 2022 Nobel Prize in chemistry[1] was awarded to scientists Carolyn R. Bertozzi, Morten Meldal and K. Barry Sharpless for their development of click chemistry and bioorthogonal chemistry.
These techniques have been used in a number of sectors, including delivering treatments[2] that can kill cancer cells without perturbing healthy cells as well as sustainably and quickly producing large amounts of polymers to build materials. One click chemistry-based drug is currently undergoing phase 2 clinical trials[3]. Bertozzi is a scientific adviser of the company developing the drug.
We asked chemistry Ph.D. candidate Heyang (Peter) Zhang[4] of the Lin Lab[5] at the University at Buffalo to talk about how these techniques figure in his own research and how they have transformed his field and other industries.
1. How does click and bioorthogonal chemistry work?
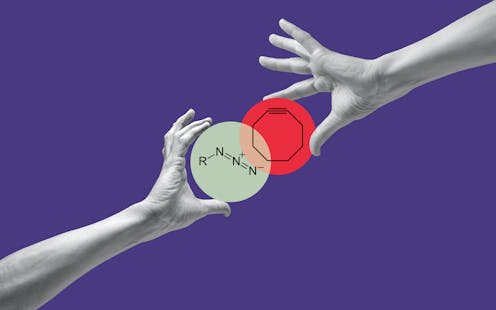
Click chemistry[6], as the name suggests, is a way of building molecules like snapping Lego blocks together. It takes two molecules to click, so researchers refer to each one as click partners.
K. Barry Sharpless and Morten Meldal independently discovered that azide[7], a high-energy molecule with three nitrogens bonded together, and alkyne[8], a relatively inert and naturally rare molecule with two carbons triple-bonded together, are great click partners in the presence of a copper catalyst[9]. They found that the copper catalyst can bring the two pieces together in an optimal arrangement that snaps them together. Prior to this technique, researchers did not have a way to quickly and precisely make new molecules under accessible conditions, like using water as a solvent at room temperature.
Chemical biologists quickly realized that click reactions can be a fantastic way to probe living systems like cells because they produce little to no toxic byproducts and can happen quickly. However, the copper catalyst is itself toxic to living systems.
Carolyn Bertozzi devised a workaround for this issue by removing the copper catalyst from the reaction[11]. She did this by placing the alkyne into a ring structure, which drives the reaction forward using the ring strain produced from molecules forced into a cyclical shape. These bioorthogonal reactions, or reactions that happen “parallel” to the chemical environment of the cell, can occur in cells without perturbing their normal chemistry.
2. How do you use this chemistry in your work?
In an interview[12], Carolyn Bertozzi stated that the next steps for bioorthogonal chemistry are to find new reactions and applications for it. Our lab’s research focuses exactly on that.
My colleagues and I apply this technique to track molecules we are interested in as they naturally behave in a cell. In a living cell, we were able to add a probe to a receptor[13] that plays a role in a number of cellular processes.
Carolyn Bertozzi is one of the winners of the 2022 Nobel Prize in chemistry.To find new reactions, our lab has spent the last 15 years to push how fast bioorthogonal reactions can run[14]. Speed is important because many molecules in living organisms are present in low concentrations, and using too much of the chemicals required for the reaction can be toxic for the cell. The faster the reaction, the fewer the unwanted side reactions.
We pioneered another way to achieve click and bioorthogonal reactions with even faster speed. Instead of using an azide and an alkyne like the Nobel Prize winners did originally, we used two other molecules that join together when a light is shined on them. With this technique, we are able to add molecules to the surface of a live cell in as little as 15 seconds[15]. We can then observe how a particular structure on a cell functions in its natural environment, or detect how it changes when exposing it to drugs or other substances. Researchers can then more easily test how cells react to potential treatments.
Currently, we are working to develop a new method of triggering these reactions without light. We are actively working on using bioorthogonal chemistry to improve PET imaging to screen and monitor tumors.
3. Why are these techniques so important to your field?
Prior to click and bioorthogonal chemistry, there was no way of visualizing molecules in living cells in their natural state.
As an analogy, imagine you needed to find a specific dollar bill with the serial number 01234567. That would be a pretty daunting task. It would require you to go through every dollar you can get your hands on and verify whether the serial number is the one you are looking for.
Tracking molecules in our body is just as hard, if not more. Because biological environments are so complex, it was previously impossible to add a probe to just the molecule of interest without accidentally tagging something else, or worse, altering the normal chemistry of the cell. With bioorthogonal reactions, however, researchers can essentially add a GPS tracker to the molecule without affecting the rest of the cell.
References
- ^ 2022 Nobel Prize in chemistry (www.nobelprize.org)
- ^ delivering treatments (www.statnews.com)
- ^ phase 2 clinical trials (clinicaltrials.gov)
- ^ Heyang (Peter) Zhang (scholar.google.com)
- ^ Lin Lab (lin.chem.buffalo.edu)
- ^ Click chemistry (doi.org)
- ^ azide (ehs.stanford.edu)
- ^ alkyne (www.angelo.edu)
- ^ presence of a copper catalyst (doi.org)
- ^ Cliu89/Wikimedia Commons (commons.wikimedia.org)
- ^ removing the copper catalyst from the reaction (doi.org)
- ^ an interview (youtu.be)
- ^ add a probe to a receptor (doi.org)
- ^ push how fast bioorthogonal reactions can run (doi.org)
- ^ as little as 15 seconds (doi.org)
- ^ Rossin 2018 (Nature Communications) (doi.org)
- ^ CC BY-NC-ND (creativecommons.org)
Authors: Heyang (Peter) Zhang, PhD Candidate in Chemistry, University at Buffalo

