Blood in your veins is not blue – here's why it's always red
- Written by Marisia Fikiet, Ph.D. Student in Chemistry, University at Albany, State University of New York
Whenever you see blood outside your body, it looks red. Why?
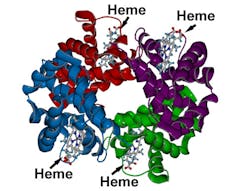 Heme is the part of the hemoglobin molecule that latches onto oxygen and then releases it to tissues around the body.
Waikwanlai, CC BY[1][2]
Heme is the part of the hemoglobin molecule that latches onto oxygen and then releases it to tissues around the body.
Waikwanlai, CC BY[1][2]
Human blood is red because of the protein hemoglobin, which contains a red-colored compound called heme that’s crucial for carrying oxygen through your bloodstream. Heme contains an iron atom which binds to oxygen[3]; it’s this molecule that transports oxygen from your lungs to other parts of the body.
Chemicals appear particular colors to our eyes based on the wavelengths of light they reflect. Hemoglobin bound to oxygen absorbs blue-green light, which means that it reflects red-orange light[4] into our eyes, appearing red. That’s why blood turns bright cherry red when oxygen binds to its iron. Without oxygen connected, blood is a darker red color[5].
Carbon monoxide, a potentially deadly gas, can also bind to heme[6], with a bond around 200 times stronger than that of oxygen. With carbon monoxide in place, oxygen can’t bind to hemoglobin, which can lead to death. Because the carbon monoxide doesn’t let go of the heme[7], your blood stays cherry red, sometimes making a victim of carbon monoxide poisoning appear rosy-cheeked even in death.
 People with pale skin may think their blood is blue inside the body.
eltpics, CC BY-NC[8][9]
People with pale skin may think their blood is blue inside the body.
eltpics, CC BY-NC[8][9]
Sometimes blood can look blue through our skin[10]. Maybe you’ve heard that blood is blue in our veins because when headed back to the lungs, it lacks oxygen. But this is wrong; human blood is never blue. The bluish color of veins is only an optical illusion. Blue light does not penetrate as far into tissue as red light. If the blood vessel is sufficiently deep, your eyes see more blue than red reflected light due to the blood’s partial absorption of red wavelengths.
But blue blood does exist elsewhere in the animal world. It’s common in animals such as squid and horseshoe crabs, whose blood relies on a chemical called hemocyanin, which contains a copper atom[11], to carry oxygen. Green, clear and even purple blood are seen in other animals[12]. Each of these different blood types uses a different molecule to carry oxygen rather than the hemoglobin we use.
 Horseshoe crabs’ blue blood has become an important raw material for the pharmaceutical industry.
AP Photo/Steve Helber[13]
Horseshoe crabs’ blue blood has become an important raw material for the pharmaceutical industry.
AP Photo/Steve Helber[13]
Despite exceptions, the majority of blood from animals is red. But that doesn’t mean it’s exactly the same as what courses through our veins. There are many variations of hemoglobin present in different species, which allows scientists to distinguish blood samples[14] from various animals.
Over time, spilled blood that starts out red turns darker and darker as it dries and its hemoglobin breaks down into a compound called methemoglobin. As time passes, dried blood continues to change, growing even darker thanks to another compound called hemichrome. This continual chemical and color change allows forensic scientists to determine the time[15] a blood drop was left at a crime scene.
In our lab[16], we’re developing methods that look at the ratio of the different compounds that hemoglobin breaks down into. Then using computer modeling we can estimate the time since the blood was deposited[17] to help investigators determine if a blood stain is relevant to a crime. If the blood is a year old, it might not be important to a crime committed yesterday.
References
- ^ Waikwanlai (commons.wikimedia.org)
- ^ CC BY (creativecommons.org)
- ^ Heme contains an iron atom which binds to oxygen (store.macmillanlearning.com)
- ^ reflects red-orange light (doi.org)
- ^ darker red color (www.acs.org)
- ^ can also bind to heme (www.ncbi.nlm.nih.gov)
- ^ carbon monoxide doesn’t let go of the heme (doi.org)
- ^ eltpics (www.flickr.com)
- ^ CC BY-NC (creativecommons.org)
- ^ blue through our skin (doi.org)
- ^ contains a copper atom (www.acs.org)
- ^ seen in other animals (news.nationalgeographic.com)
- ^ AP Photo/Steve Helber (www.apimages.com)
- ^ distinguish blood samples (doi.org)
- ^ allows forensic scientists to determine the time (doi.org)
- ^ In our lab (sites.google.com)
- ^ estimate the time since the blood was deposited (doi.org)
Authors: Marisia Fikiet, Ph.D. Student in Chemistry, University at Albany, State University of New York
Read more http://theconversation.com/blood-in-your-veins-is-not-blue-heres-why-its-always-red-97064

