Labs are experimenting with new – but unproven – methods to create a coronavirus vaccine fast
- Written by Jean Peccoud, Professor, Abell Chair in Synthetic Biology, Colorado State University
The coronavirus has ground social, economic and educational exchanges to a halt around the world. For now, public health officials are relying on tools like social distancing to minimize the harm of the virus, but in the long term, a COVID-19 vaccine is the best hope of a return to normalcy.
It normally takes a few years to development a vaccine[1], but in the face of the coronavirus, biotechnology companies and regulatory agencies are taking aggressive steps to make a COVID-19 vaccine widely available sooner than that.
I study biomanufacturing and synthetic biology[2], and it is fascinating to watch this unprecedented effort push at the limits of vaccine development. Public and private labs around the world are pursuing cutting-edge vaccine engineering strategies that have never been tested on such a large scale. If these efforts succeed, the vaccine would become an essential tool to fight or prevent future COVID epidemics.
How vaccines work
The first time the body is exposed to a new virus, it takes weeks to build antibodies and other defense mechanisms[3] that will fight it off. This gives the virus plenty of time to replicate and make someone sick.
However, the immune system has memory. If it has encountered a virus before, the body can quickly deploy its defenses against the invader and neutralize the virus before a full infection develops.
This is the idea behind vaccines: give the body an opportunity to build defenses against a virus it may encounter in the future. Not all vaccines produce the same level of immunological preparedness[4] – the stronger the initial immune response, the better the vaccine – but some preparation is better than none.
 Vaccines have been around for nearly 150 years, and until recently, the science hasn’t fundamentally changed.
AP Photo/File[5]
Vaccines have been around for nearly 150 years, and until recently, the science hasn’t fundamentally changed.
AP Photo/File[5]
The traditional way of developing a vaccine is to grow and inject patients with inactivated viruses. These don’t make you sick, but once exposed to these “dead” viruses, the immune system will have the weapons to fight off that virus in the future, if it needs to.
Unfortunately, figuring out how to grow a new virus on an industrial scale is complicated, and once done, the process itself is often slow, difficult and potentially risky. For example, the flu vaccine is produced by growing the virus in millions of chicken eggs. The process takes four months. In addition, when dealing with a virus for which there is no drug or vaccine, it is safer to avoid growing it in large quantities for fear that it might accidentally leak out of the factory and make the situation even worse than it already is.
With the coronavirus literally making time a matter of life and death, nearly 50 public and private labs[6] are turning to newer, safer and faster methods to develop a coronavirus vaccine.
Protein-based vaccines
Rather than injecting the whole virus, it is possible to vaccinate a person with a single virus component. The pieces most commonly used are proteins from the surface of a virus. If a live virus enters the body, these surface proteins are easily recognized by the immune system. This approach is easier, faster and safer because the virus protein can be produced in cell cultures.
 By using proteins from the surface of the virus, it is possible to vaccinate a person without going through the complicated process of growing a dangerous virus.
ayvengo/iStock/Getty Images Plus via Getty Images[7]
By using proteins from the surface of the virus, it is possible to vaccinate a person without going through the complicated process of growing a dangerous virus.
ayvengo/iStock/Getty Images Plus via Getty Images[7]
Two companies, Sanofi[8] and Novawax[9], are both developing protein vaccines based on the SARS-CoV-2 spike[10] protein, the tower-shaped structures on the surface of the new coronavirus that causes COVID-19.
Protein-based vaccines, also known as recombinant vaccines, are already used to vaccinate against viral infections like HPV[11]. They are far simpler to produce compared to traditional whole-virus vaccines, but it can still take a year to develop a new process and several weeks to produce the vaccine after the manufacturing process has been developed. The world needs something faster.
Gene-based vaccines
Theoretically, the simplest and fastest way to make a vaccine would be to have a person’s own cells produce minute quantities of the viral protein that trigger an immune response. To do that researchers are turning to genetics.
The first genetic approach uses DNA. A single gene that codes for a protein from the coronavirus is injected into the patient’s cells in the hopes that a small fraction of the DNA molecules will find their way into the cell nucleus. There they would be copied into an RNA molecule which is then read by the cell to produce the viral protein. But it is difficult to get the human body to produce enough protein using this approach. Frequently, very little DNA makes it to the cell nucleus and the cell does not produce the protein in sufficient quantity to trigger a strong enough immune response.
As of yet, there are no DNA vaccines currently approved by the FDA[12] for human use and the success of this method has been limited. But there is promise. In 2016, several groups developed candidate Zika vaccines using this technology[13] and at least one company, INOVIO Pharmaceuticals, Inc.[14] is developing INO-4800[15], a DNA vaccine candidate for the coronavirus.
The bottleneck of DNA vaccines is getting the DNA to the nucleus to be transcribed into RNA. Vaccines that use RNA directly might be able to overcome this problem. Since RNA is translated into proteins as soon as it enters the cell, this approach results in stronger immune responses[16] than DNA vaccines. However, RNA breaks down faster than DNA.
This has not deterred a number of companies from trying it though. Notable in the U.S. is Moderna[17], and on March 16, the National Institutes of Health started a clinical trial[18] of Moderna’s lead coronavirus vaccine candidate, mRNA-1273.
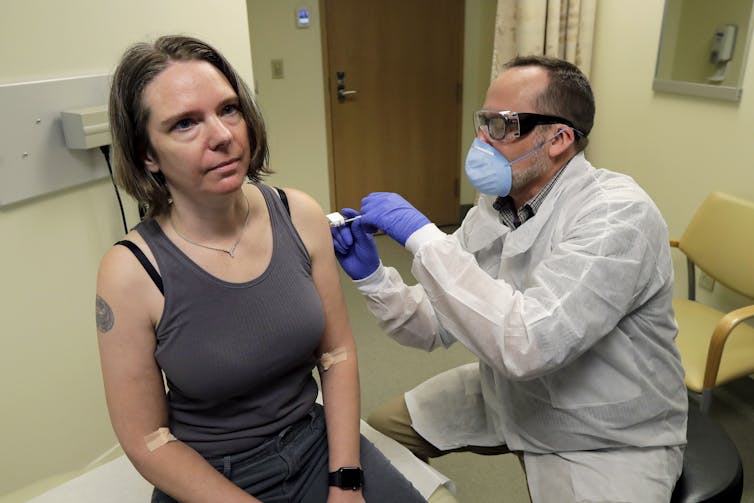 On March 16, 2020, Jennifer Haller of Seattle, Wash., became the first person to try Moderna’s experimental RNA vaccine.
AP Photo/Ted S. Warren[19]
On March 16, 2020, Jennifer Haller of Seattle, Wash., became the first person to try Moderna’s experimental RNA vaccine.
AP Photo/Ted S. Warren[19]
Manufacturing DNA and RNA relies on standardized and fairly simple processes. DNA vaccines are produced in bacteria[20] that grow overnight while RNA vaccines are produced in test tubes[21] using a biochemical reaction that only takes hours. Gene-based vaccines could be produced extremely quickly compared to traditional or protein-based vaccines.
Friendly virus vaccines
The main issue with gene-based vaccines is getting the DNA or RNA to where it needs to be. One elegant way to solve this challenge is to use a harmless virus as a delivery system. Viruses are extremely good at penetrating cells; once inside, a virus with genes from SARS-CoV-2 could use the machinery of the cell to produce proteins to trigger an immune response for the coronavirus.
This technique is being pursued by a few companies around the world. For example, Hong Kong-based CanSino Biologics[22] is inserting the coronavirus gene that codes for the spike protein into an adenovirus[23]. They used this strategy to produce the first government-approved Ebola vaccine[24], and clinical trials of an engineered adenovirus that would protect against the coronavirus have already started in China.
The production of vaccines delivered by harmless viruses is slower than producing DNA or RNA vaccines because it involves the culture of slow-growing mammal cells. However, like the production of gene-based vaccines, they rely on existing processes that take advantage of viruses that have been optimized for manufacturing.
Containing the epidemic with imperfect vaccines
While the pace of COVID-19 vaccine development is unprecedented, the timeline to mass vaccination still remains uncertain. While the large number of approaches being pursued may give the impression of desperation and confusion, it is actually reassuring. This multipronged approach is a way to hedge the vaccine development bet.
It is unlikely the first vaccines developed will be 100% effective and easy to produce on a massive scale. Realistically, researchers will develop a number of good-enough vaccines that can be produced using different kinds of manufacturing infrastructures. While these vaccines may at first have a limited efficacy[25], the diversity in manufacturing processes will allow companies to make and distribute them quickly, buying time and helping contain the current epidemic and prevent future outbreaks[26].
[Get facts about coronavirus and the latest research. Sign up for our newsletter.[27]]
References
- ^ normally takes a few years to development a vaccine (www.fda.gov)
- ^ study biomanufacturing and synthetic biology (peccoud.org)
- ^ it takes weeks to build antibodies and other defense mechanisms (www.cdc.gov)
- ^ same level of immunological preparedness (www.ncbi.nlm.nih.gov)
- ^ AP Photo/File (www.apimages.com)
- ^ nearly 50 public and private labs (www.raps.org)
- ^ ayvengo/iStock/Getty Images Plus via Getty Images (www.gettyimages.com)
- ^ Sanofi (www.pharmatimes.com)
- ^ Novawax (ir.novavax.com)
- ^ protein vaccines based on the SARS-CoV-2 spike (science.sciencemag.org)
- ^ vaccinate against viral infections like HPV (www.who.int)
- ^ no DNA vaccines currently approved by the FDA (dx.doi.org)
- ^ Zika vaccines using this technology (www.technologyreview.com)
- ^ INOVIO Pharmaceuticals, Inc. (ir.inovio.com)
- ^ INO-4800 (www.researchsquare.com)
- ^ results in stronger immune responses (doi.org)
- ^ Notable in the U.S. is Moderna (www.modernatx.com)
- ^ started a clinical trial (investors.modernatx.com)
- ^ AP Photo/Ted S. Warren (www.apimages.com)
- ^ DNA vaccines are produced in bacteria (doi.org)
- ^ RNA vaccines are produced in test tubes (doi.org)
- ^ CanSino Biologics (gmpnews.net)
- ^ adenovirus (www.cdc.gov)
- ^ first government-approved Ebola vaccine (dx.doi.org)
- ^ may at first have a limited efficacy (www.cdc.gov)
- ^ contain the current epidemic and prevent future outbreaks (doi.org)
- ^ Sign up for our newsletter. (theconversation.com)
Authors: Jean Peccoud, Professor, Abell Chair in Synthetic Biology, Colorado State University

